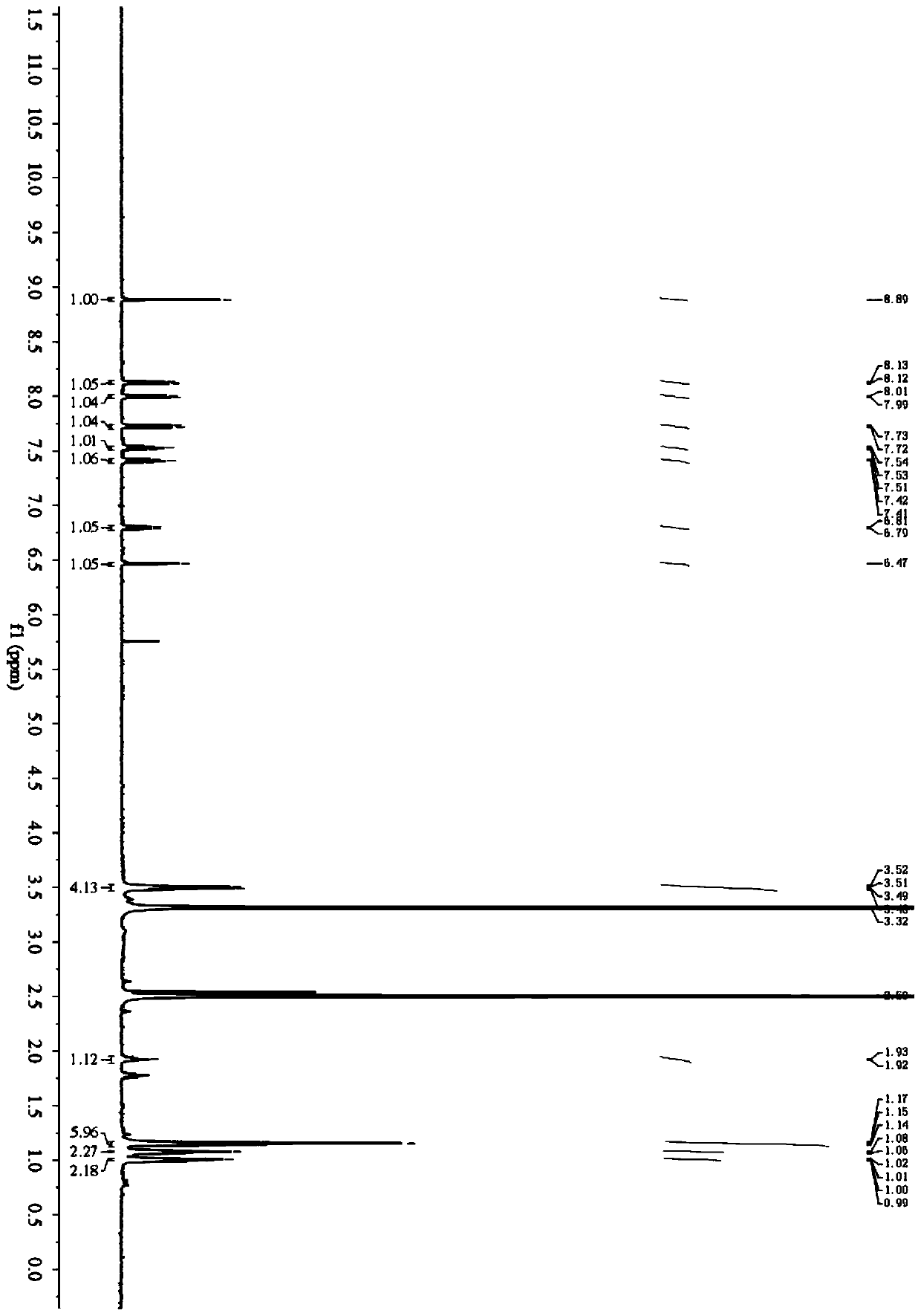
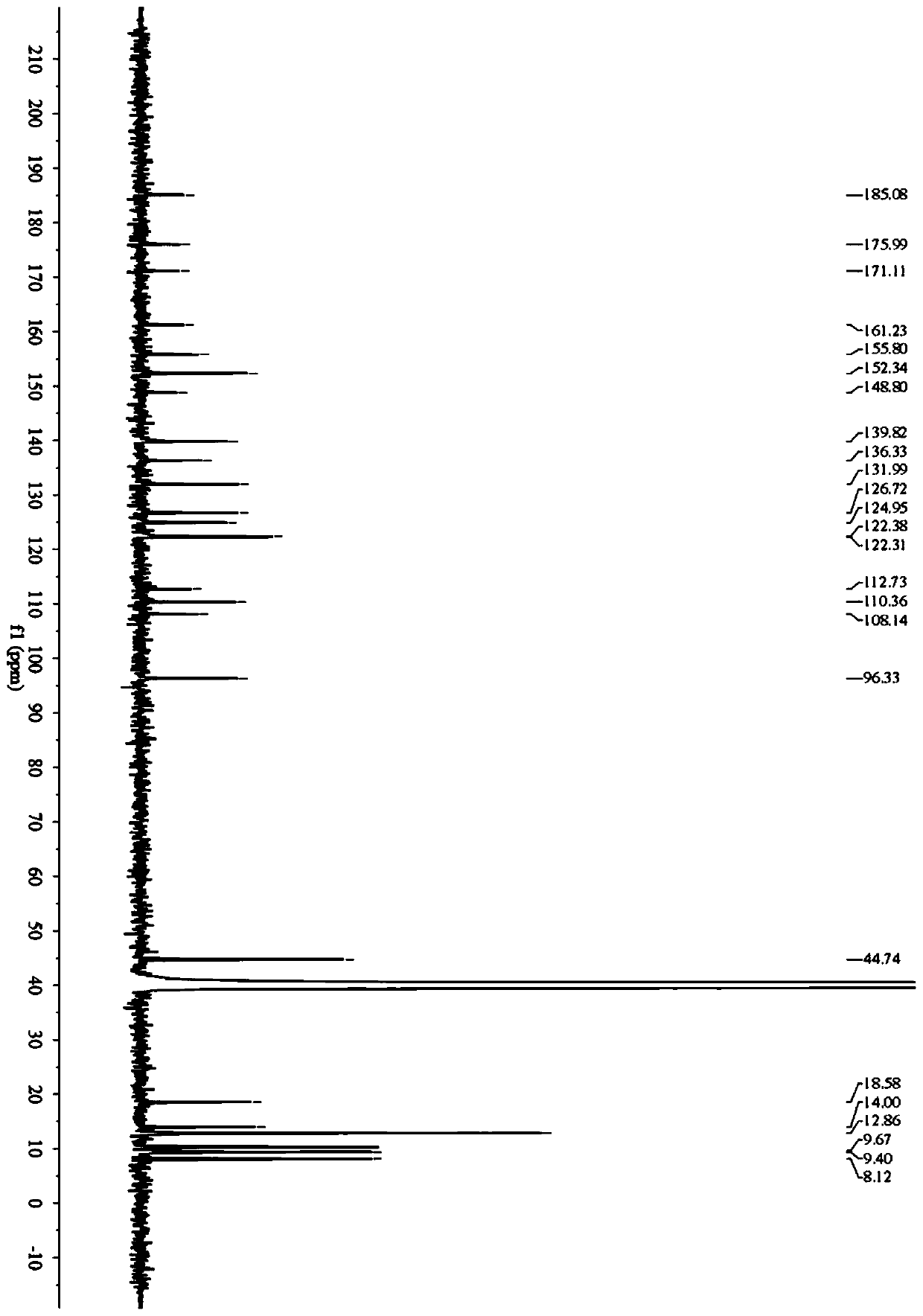
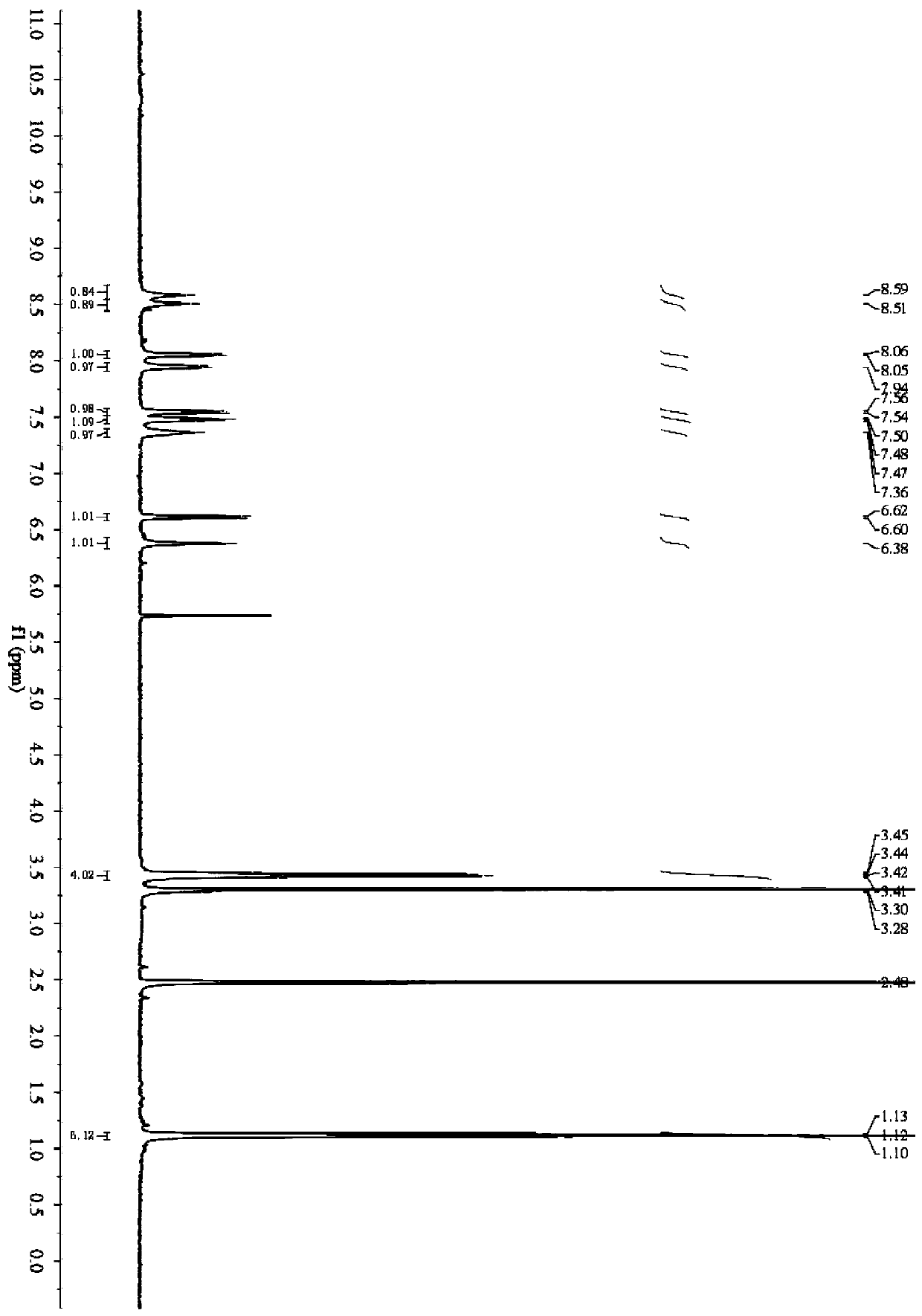
Fluorescent probe for detecting butyrylcholinesterase activity as well as synthesis method and application of fluorescent probe
A technology of butyrylcholinesterase and fluorescent probe, which is applied in the field of detection of butyrylcholinesterase activity, can solve the problems of unfavorable BChE determination, lower accuracy, lower detection sensitivity, etc., and achieve the goal of avoiding fluorescence background interference Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the synthesis of fluorescent probe
[0057] 1. Synthesis steps:
[0058] (1) Dissolve 4-(diethylamino)salicylaldehyde (0.97g, 5.0mmol) and triethylamine (1.40mL, 10.0mmol) in 20mL of anhydrous dichloromethane at 0°C, then add in Cyclopropanecarbonyl chloride (0.63 g, 6.0 mmol) in 10 mL of dichloromethane, the mixture was stirred at room temperature overnight; after quenching the reaction, the solution was washed 3 times with brine, and the organic phase was dried over anhydrous sodium sulfate and then distilled under reduced pressure The solvent was removed, and the crude product was purified by column chromatography (PE:EA=10:1), to obtain compound 1 with a yield of 83%, and the reaction process is shown in formula (1):
[0059]
[0060] (2) Weigh 1.25g (10.0mmol) 2-aminobenzenethiol and 0.8g (12.0mmol) malononitrile, dissolve in 20mL absolute ethanol, add 0.5mL acetic acid, stir and react at room temperature for 5h, extract and separate, and Pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com