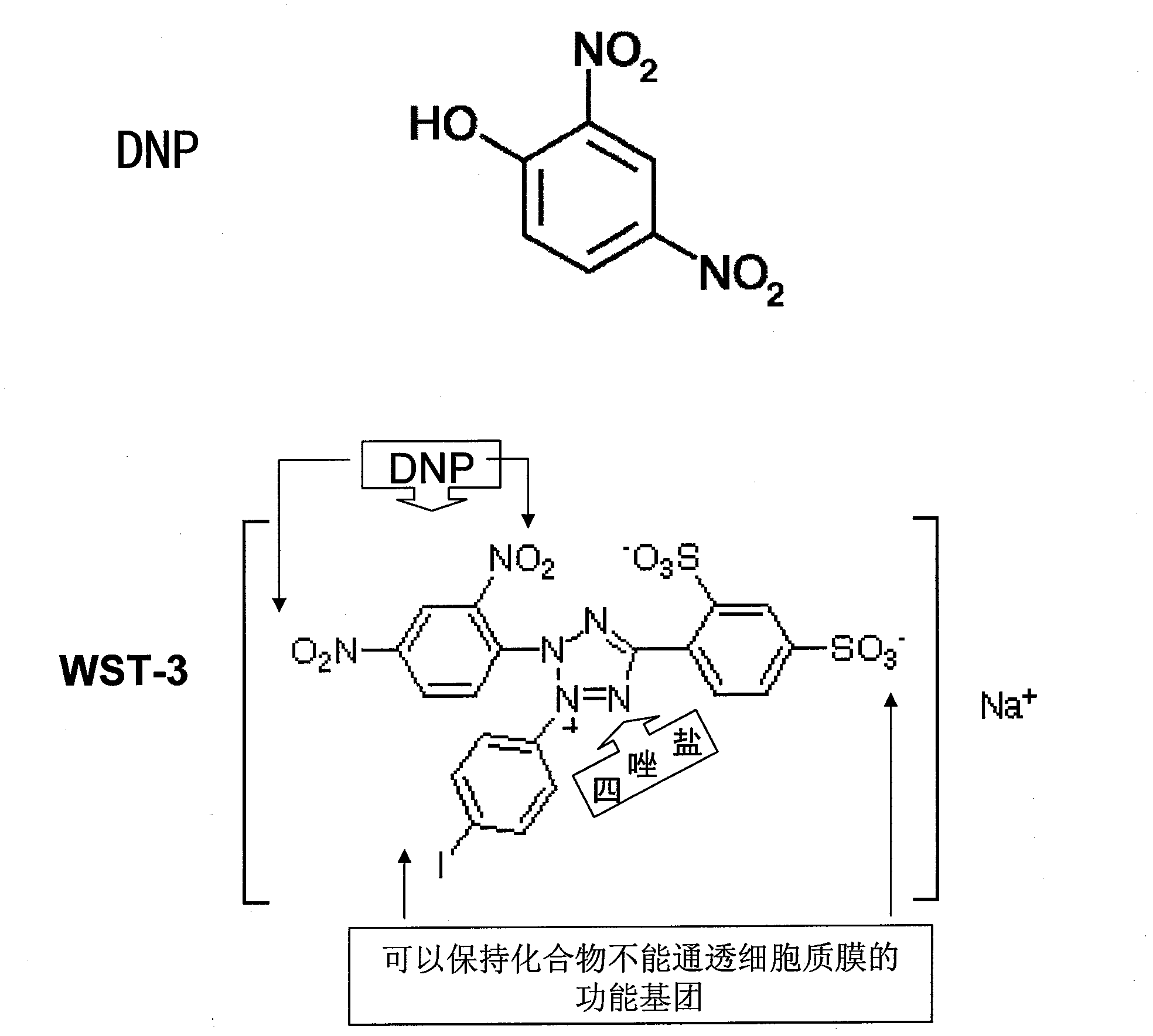
Compounds, compositions and use for anticancer therapy
A compound and drug technology, applied in the field of tumor and chemotherapy, can solve the problems of electron transfer process without tPMET and coupling of oxidative phosphorylation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0165] Example 1 mPMS dose-response relationship
[0166] Methods: A and B: HT1080 (Fig. 2A) and UM-SCC6 (Fig. 2B) cells were given different concentrations of mPMS shown in the figure, combined with 1 mMWST-1c and 10, 30 or 100 M apigenin for 4 hours, and then, changed to Normal growth medium for 24 hours. CCK8 kit was used to measure cell viability. Parallel controls were 0.12 mM mPMS, or 10% WST-1c. AP0: no apigenin control, AP10: 10M apigenin, AP30: 30M apigenin, AP100: 10M apigenin. Results: The data showed that mPMS and apigenin dose-dependently killed HT1080 and UM-SCC6 cells (Fig. 2A and B). HT1080 cells were applied with mPMS, and mPMS+WST-1, and the IC50 of mPMS combined with apigenin 100M was 5M, while the IC50 of the untreated control mPMS was 60M. SCC6 cells were applied with mPMS, and mPMS+WST-1, and the IC50 of mPMS combined with apigenin 100M was 30M, while the IC50 of the untreated control mPMS was 80M.
example 2
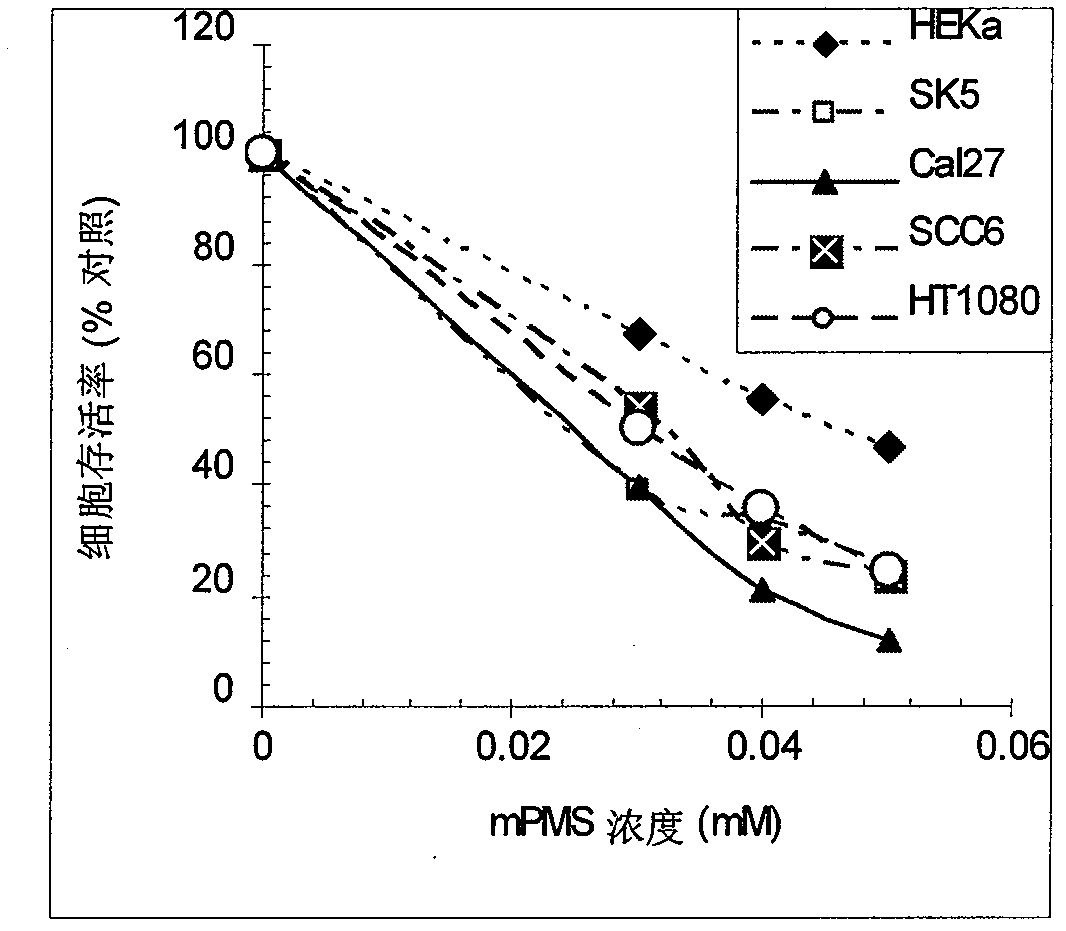
[0167] Example 2 Dose-response relationship of different cells to mPNS
[0168] Non-cancerous human keratinocytes (HEKA), human malonoma cell line SK-MEL-5 (SK5), human head and neck tumor cell line Cal27 (Cal27), and UM-SCC6 (SCC6) cells, and human soft tissue sarcoma cell line HT1080 (HT1080) were cultured with mPMS 30, 40 or 50M for 4 hours and then in normal growth medium for an additional 24 hours. CCK8 kit was used to measure cell viability. The data showed dose-dependent cell death and sensitivity differences to mPMS among different cell lines to mPMS treatment from each cell line (Figure 3). Among them, non-tumor-derived primary cultured HEKA cells were the least sensitive to mPMS, with an IC50 of 50M mPMS, while its IC50 was 20 and 30M from the IC50 of Cal27, UM-SCC6 and HT1080 cell mPMS, respectively.
example 3
[0169] Example 3 The effect of combined treatment of apigenin and WST-3 in inducing cancer cell death,
[0170] Methods: UM-SCC6, HT1080, Cal27, SK-MEL-5, and HEKA cells were administered alone or in combination with 50 or 100M WST-3 or 10 or 30M apigenin, or in combination with different concentrations of WST-3 and apigenin 4 hours, untreated cells were used as controls, and then changed to normal growth medium for 24 hours. The cell viability was measured using CCK8 kit. Data corrected as % of untreated control cells.
[0171] Results: A: Response of different cells to treatment with WST-3, apigenin, and combination. Application of 50M WST-3 (WST-3) or 30M apigenin (Apigenin) alone to each tested cell line showed no or less cell death effect compared to untreated cells (CTRL). Combination of WST-3 and apigenin (celery + WST-3) leads to synergistic cell death in all four tested human cancer cell lines of SK-MEL-5, Cal27, UM's SCC6 and HT1080, but not tumor human cells Onl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com