Enhancement of cellular production through mechanotransduction
A technology of cell and cell adhesion, applied in stress-stimulated microbial growth methods, biochemical instruments, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0069] 1. Cell culture
[0070] CHO cells producing recombinant, glycosylated antibodies (eg, ABT-874) are used. ABT-874 producing floating CHO cells were adapted for growth in serum. Cells were cultured on cell culture plates under the nominal growth conditions used in fed-batch reactors.
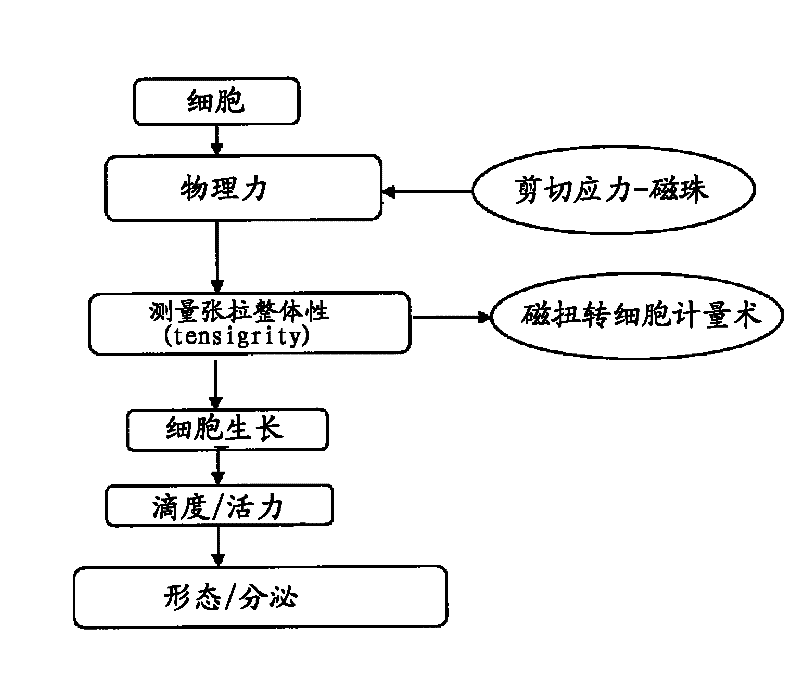
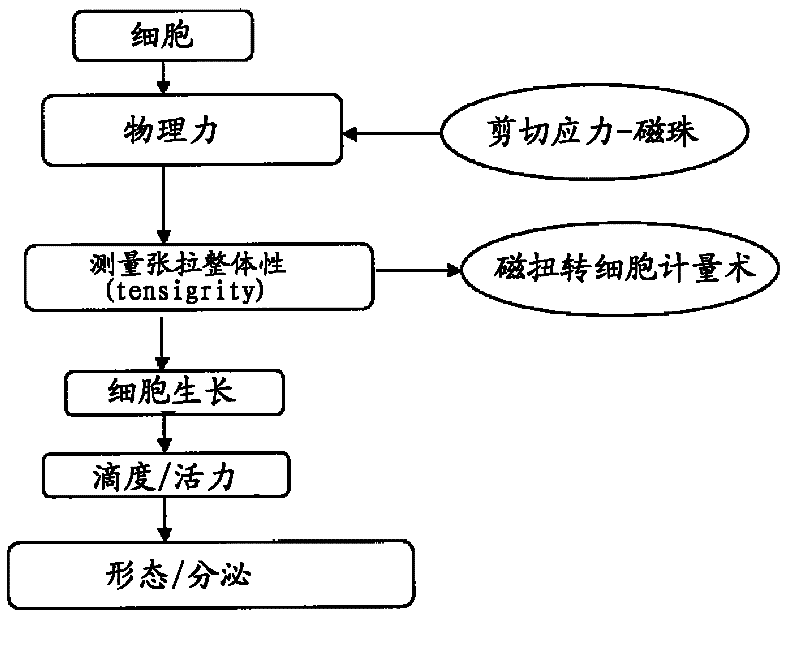
[0071] 2 Generation of tensegrity forces on cells
[0072] Cells were introduced into the tensegrity model using two different techniques as described in sections 2.1 and 2.2 below. Cell-based assays were used to optimize and evaluate changes in mechanotransduction induced by these forces.
[0073] 2.1 Optical magnetic torsional cytometry
[0074] At various time points, cells are subjected to controlled mechanical shear stress applied directly to the cell surface. Shear stress (torque) is applied to the surface using membrane-bound ferromagnetic beads (1-6 μm in diameter) coated with anti-CHO antibody that adheres to the cell's cytoskeleton. The beads are magnetized in one direction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com