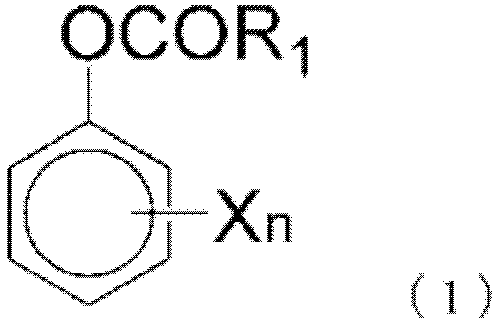
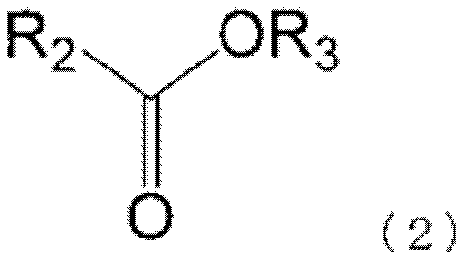
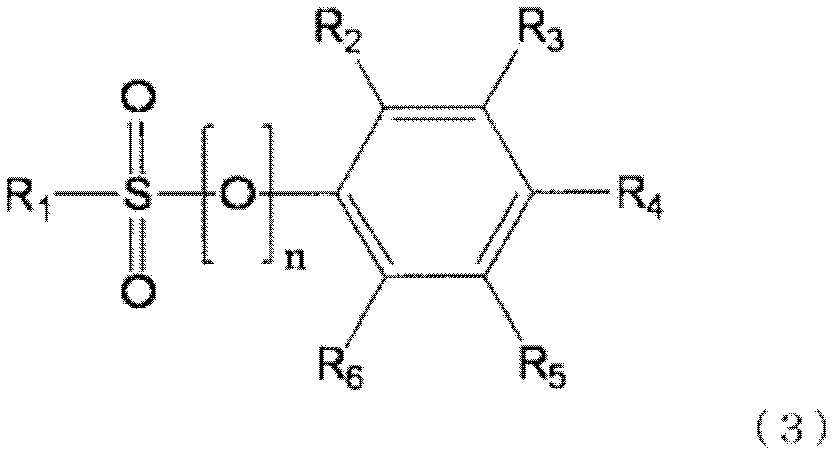
Non-aqueous electrolytic solution, and non-aqueous electrolyte battery comprising same
A non-aqueous electrolyte, non-aqueous technology, applied in non-aqueous electrolyte batteries, electrolytes, lithium batteries, etc., can solve problems such as no clear records, and achieve the effect of improving continuous charging characteristics and improving overcharging characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0398] In a mixed solvent of ethylene carbonate (EC) as a saturated cyclic carbonate, dimethyl carbonate (DMC) as a chain carbonate, and ethyl methyl carbonate (EMC) (mixing volume ratio 2:7:1) In the 1mol / L ratio, LiPF as the electrolyte 6 After dissolving, 4-fluorophenyl acetate and vinylene carbonate (VC) were added so as to make them 2% by weight with respect to the total weight of the electrolytic solution, respectively, to prepare a non-aqueous electrolytic solution. Using the obtained non-aqueous electrolytic solution, a lithium secondary battery was manufactured according to the above-mentioned method, and a capacity evaluation test, an overcharge characteristic evaluation test, and a storage characteristic evaluation test were performed. The results are shown in Table 1.
Embodiment 2
[0400] In a mixed solvent of ethylene carbonate (EC), propylene carbonate (PC) as a saturated cyclic carbonate, and dimethyl carbonate (DMC) as a chain carbonate (mixing volume ratio 2:1:7 ), LiPF as electrolyte is made at a ratio of 1mol / L 6 After dissolving, 4-fluorophenyl acetate and vinylene carbonate (VC) were added so as to make them 2% by weight with respect to the total weight of the electrolytic solution, respectively, to prepare a non-aqueous electrolytic solution. Using the obtained non-aqueous electrolytic solution, a lithium secondary battery was manufactured according to the above-mentioned method, and a capacity evaluation test, an overcharge characteristic evaluation test, and a storage characteristic evaluation test were performed. The results are shown in Table 1.
Embodiment 3
[0402] In a mixed solvent of ethylene carbonate (EC) as a saturated cyclic carbonate, dimethyl carbonate (DMC) as a chain carbonate, and ethyl methyl carbonate (EMC) (mixing volume ratio 2:7:1) In the 1mol / L ratio, LiPF as the electrolyte 6 After dissolving, 4-fluorophenyl acetate and vinylene carbonate (VC) were added so as to make them respectively 4% by weight and 2% by weight relative to the total weight of the electrolyte solution to prepare a non-aqueous electrolyte solution. Using the obtained non-aqueous electrolytic solution, a lithium secondary battery was manufactured according to the above-mentioned method, and a capacity evaluation test, an overcharge characteristic evaluation test, and a storage characteristic evaluation test were performed. The results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com