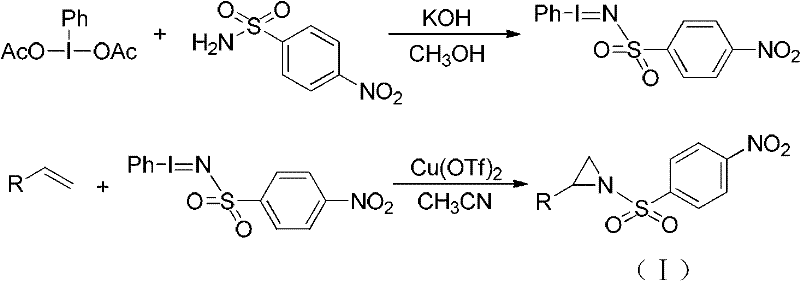
2, 3, 4, 7-tetrahydro-1h-nitrogen azepine compound and preparation method and application thereof
A compound, azapine technology, applied in the field of organic synthesis, can solve problems such as rare synthetic methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
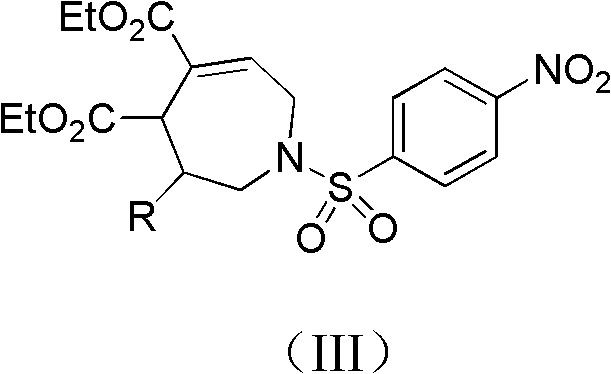
[0027] Example 1, 1-p-nitrobenzenesulfonyl-3-phenyl-2,3,4,7-tetrahydro-1H-azepine-4,5-dicarboxylic acid diethyl represented by formula (IV) Synthesis of esters
[0028]
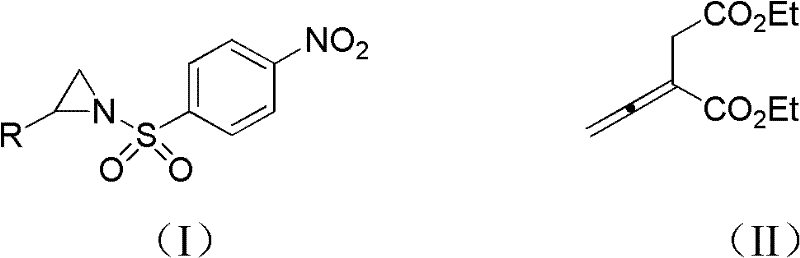
[0029] Compound 1-p-nitrobenzenesulfonyl-2-phenylaziridine (0.125mmol), 5mL dichloromethane and 0.0297g formula (II) shown in 0.0381g formula (II) 2-vinylsuccinate Diethyl diethyl ester (0.150mmol) was put into a 15mL Shrek tube dried in an oven, and the phosphine catalyst propyldiphenylphosphine (0.125mmol) was added, and the cycloaddition reaction was carried out with stirring. In this reaction system, 1-p The molar ratio of nitrobenzenesulfonyl-2-phenylaziridine to 2-vinylsuccinic acid diethyl ester is 1:1.2, and ethyldiphenylphosphine accounts for 1-p-nitrobenzenesulfonyl-2-benzene The molar percentage of aziridine is 100%, stirred at 25 DEG C for 48 hours, passed through the column after concentrating the reaction solution with a rotary evaporator (ethyl acetate: petroleum ether=1: 8, v / v), and obtai...
Embodiment 2
[0031] Example 2, 1-p-nitrobenzenesulfonyl-3-o-bromophenyl-2,3,4,7-tetrahydro-1H-azepine-4,5-dicarboxylic acid represented by formula (V) Synthesis of Diethyl Ester
[0032]
[0033]Compound 1-p-nitrobenzenesulfonyl-2-o-bromophenylaziridine (0.125mmol) shown in 0.0481g formula (I), 5mL dichloromethane and 0.0495g compound 2-ethylene shown in formula (II) Diethyl succinate (0.250mmol) was put into a 15mL Shrek tube dried in an oven, the phosphine catalyst ethyldiphenylphosphine (0.0625mmol) was added, and the cycloaddition reaction was carried out with stirring. In the reaction system, 1 - The molar ratio of p-nitrobenzenesulfonyl-2-o-bromophenylaziridine to 2-vinylsuccinic acid diethyl ester is 1:2, and ethyldiphenylphosphine accounts for 1-p-nitrobenzenesulfonyl The molar percentage of -2-o-bromophenylaziridine is 50%, stirred at 30°C for 72 hours, concentrated the reaction solution with a rotary evaporator, and passed the column (ethyl acetate:petroleum ether=1:8, v / v)...
Embodiment 3
[0035] Example 3, 1-p-nitrobenzenesulfonyl-3-m-methylphenyl-2,3,4,7-tetrahydro-1H-azepine-4,5-dicarboxylic acid represented by formula (VI) Synthesis of Diethyl Acetate
[0036]
[0037] Compound 1-p-nitrobenzenesulfonyl-2-m-tolyl aziridine (0.125mmol) shown in 0.0399g formula (I), 5mL dichloromethane and 0.0742g compound 2-vinyl shown in formula (II) Diethyl succinate (0.375mmol) was put into a 15mL Shrek tube dried in an oven, the phosphine catalyst butyldiphenylphosphine (0.025mmol) was added, and the cycloaddition reaction was carried out with stirring. In the reaction system, 1- The molar ratio of p-nitrobenzenesulfonyl-2-p-tolyl aziridine to 2-vinylsuccinic acid diethyl ester is 1:3, and butyldiphenylphosphine accounts for 1-p-nitrobenzenesulfonyl-2 -The molar percentage of p-tolyl aziridine is 20%, stirred at 40°C for 48 hours, concentrated the reaction solution with a rotary evaporator and passed it through the column (ethyl acetate:petroleum ether=1:8, v / v), 18.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com