Method for synthesizing heparin ester
A synthesis method and esterification technology, which are applied in the field of synthesis of heparin esterification, to achieve the effects of easy control of the reaction process, convenient operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
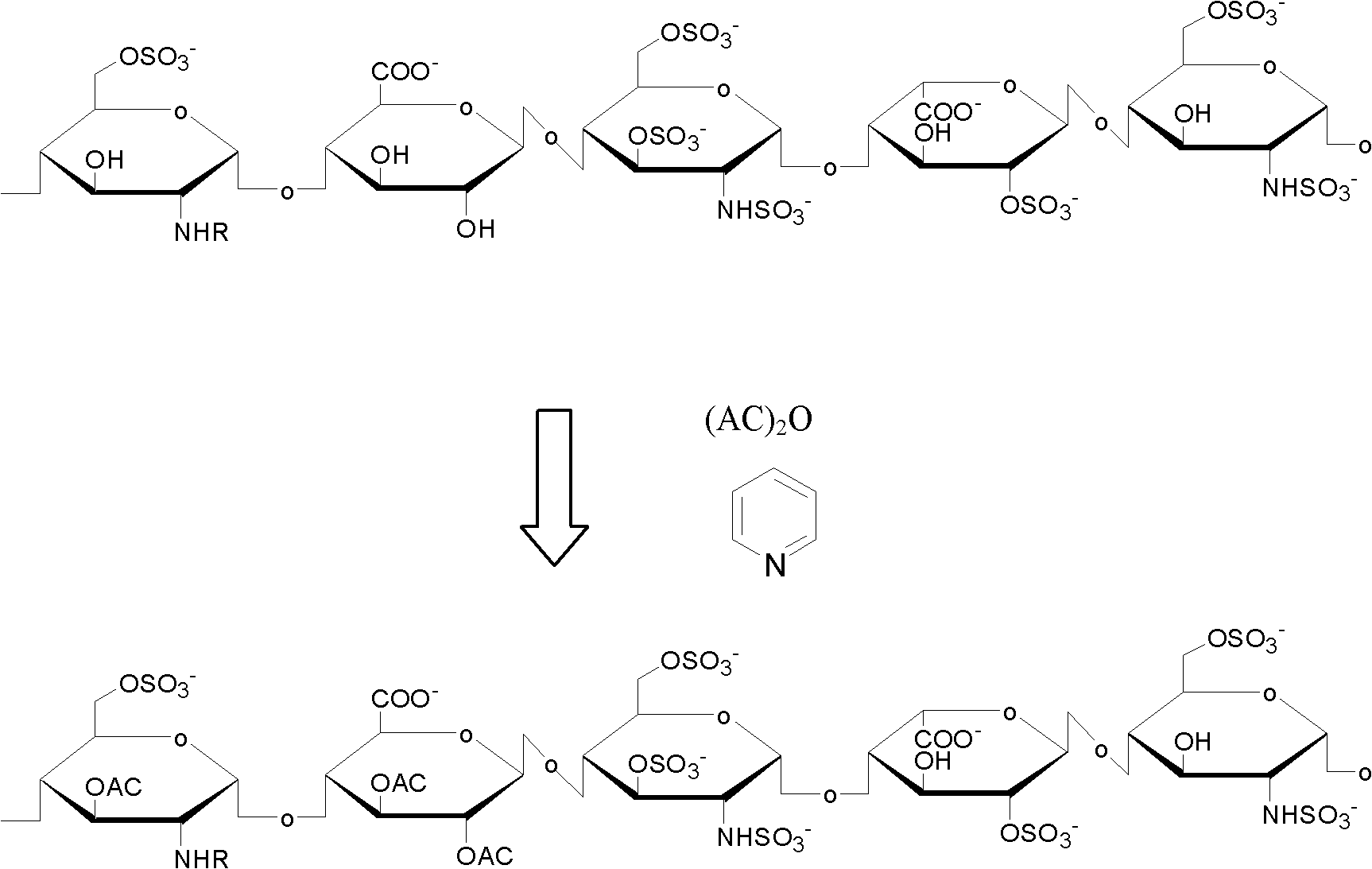
[0036] (1) Take 12g of low molecular weight heparin sodium and 12g of pyridine, add them into a dry reactor and mix them uniformly to obtain a mixture;
[0037] (2) Add 12 g of acetic anhydride to the above mixture, and stir for 10 hours at room temperature, normal pressure, and under the protection of an inert gas to carry out the esterification reaction;
[0038] (3) After adding 12 g of absolute ethanol to stop the reaction, filter to obtain a white powder, dissolve the white powder in deionized water, put it into a dialysis bag for dialysis for 12 hours, and freeze-dry to obtain the target compound, namely heparin ester.
[0039] The yield of the obtained heparin ester is 80%, the purity detected by high performance liquid chromatography is 98%, the average molecular weight is 12KDa, the heavy metal is ≤3ppm, and the bacterial endotoxin is ≤0.11IU / heparin unit.
[0040] The prepared heparin ester can be treated with an alkaline solution to make the pH value in the PBS buff...
Embodiment 2
[0043] (1) Take 12 g of heparin sodium and grind it into powder, 120 g of pyridine, add it into a dry reactor and mix it evenly to obtain a mixture;
[0044] (2) 120 g of propionic anhydride was added to the above mixture, and stirred for 10 hours at room temperature, normal pressure, and under the protection of an inert gas to carry out the esterification reaction;
[0045](3) After adding 120 g of absolute ethanol to stop the reaction, filter to obtain a white powder, then wash twice with absolute ethanol, dissolve the white powder in 600 ml of deionized water, put it into a dialysis bag for dialysis for 48 hours, and freeze-dry to obtain the target Compound, namely heparin ester.
[0046] The yield of the obtained heparin ester is 79%, the purity detected by high performance liquid chromatography is 98%, the average molecular weight is 8000Da, the heavy metal is ≤3ppm, and the bacterial endotoxin is ≤0.11IU / heparin unit.
[0047] The prepared heparin ester can be treated w...
Embodiment 3
[0050] (1) Take 12 g of heparin sodium and grind it into powder, and 60 g of aminopyridine, add them into a dry reactor and mix them uniformly to obtain a mixture;
[0051] (2) Add 60 g of hexanoic anhydride to the above mixture, and stir for 6 hours at 0° C. under normal pressure and under the protection of an inert gas to carry out esterification reaction;
[0052] (3) After adding 60 g of absolute ethanol to stop the reaction, filter to obtain a white powder, and freeze-dry to obtain the target compound, ie heparin ester.
[0053] The yield of the obtained heparin ester is 80%, the purity detected by high performance liquid chromatography is 98%, the average molecular weight is: 5000Da, the heavy metal is ≤3ppm, and the bacterial endotoxin is ≤0.11IU / heparin unit.
[0054] The prepared heparin ester can be treated with an alkaline solution to make the pH value in the PBS buffer solution 6.0-7.0, which meets the needs of human medicine.
[0055] The main components of the f...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap