Method for separating lead and zinc from polymetallic copper sulfide ore
A copper sulfide ore and multi-metal technology, applied in the field of zinc, separation and removal of lead, can solve the problem that lead and zinc are difficult to separate from copper, and achieve the effect of improving electrolysis efficiency and preventing slurry precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
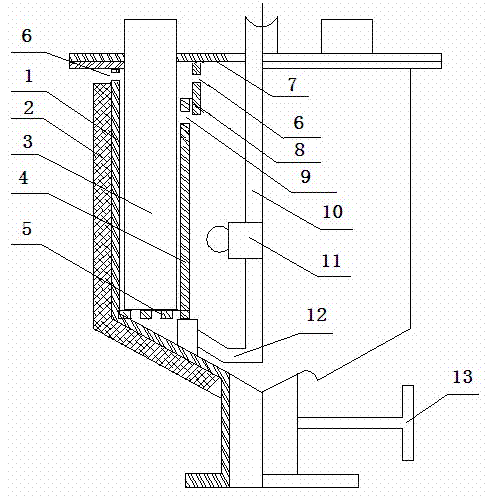
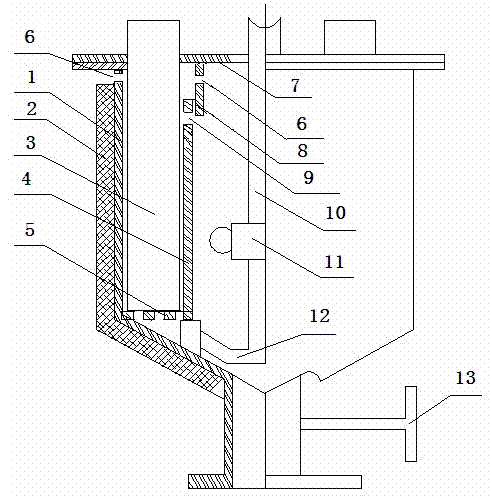
[0031] The method for separating and removing lead and zinc from the polymetallic copper sulfide ore enumerated by the present embodiment comprises the following steps: 80 objects containing Cu19.0%, Pb10.77%, and 25Kg of copper sulfide ore powder of Zn7.2% are placed in In the electrolytic cell, it is electrolyzed in a NaCl+HCl electrolyte system with a volume of 125L (the mass solid-to-liquid ratio of copper sulfide ore powder and NaCl+HCl electrolyte system is 1:4); then the electrolyte is separated from the copper ore powder, thereby Removal of lead and zinc from copper ore fines. In the above method, the molar concentration of NaCl in the electrolytic system is 3 mol / L; the dispersant NaCl is added to the NaCl+HCl electrolyte system 5 P 3 o 10 (sodium tripolyphosphate), Na 5 P 3 o 10 The dosage is 125g.
[0032]The electrolysis parameters are: the electrolysis temperature is 60°C, the pH value is 1.5, and the current density is 200 A / m 2 , the cell voltage is 3.0V....
Embodiment 2
[0048] The method for separating and removing lead and zinc from polymetallic copper sulfide ore enumerated by the present embodiment comprises the following steps: 200 meshes containing Cu19.0%, Pb10.77%, Zn7.2% copper sulfide ore powder 25Kg is placed In the electrolyzer used in the present invention, electrolyze in the NaCl+HCl electrolyte system that volume is 150L (the mass solid-liquid ratio of copper sulfide ore powder and NaCl+HCl electrolyte system is 1:5); Then electrolytic solution and copper ore powder Separation, thereby lead and zinc are removed from copper ore powder; In the above method, the molar concentration of NaCl in the electrolytic system is 3.5 mol / L; NaCl+HCl electrolyte system is added with dispersant Na 4 P 2 o 7 (sodium pyrophosphate), Na 4 P 2 o 7 The dosage is 150g.
[0049] The electrolysis parameters are controlled as follows: the electrolysis temperature is 80°C, the pH value is 3.5, and the current density is 800 A / m 2 , the cell voltage...
Embodiment 3
[0055] The method for separating and removing lead and zinc from polymetallic copper sulfide ore enumerated by the present embodiment comprises the following steps: first 120 objects containing Cu19.0%, Pb10.77%, and 25Kg of copper sulfide ore powder of Zn7.2% are placed In an electrolytic cell (the electrolytic cell is the same as in Example 2), electrolyze in a NaCl+HCl electrolyte system with a volume of 115L (the mass solid-to-liquid ratio of copper sulfide ore powder and NaCl+HCl electrolyte system is 1:3.6); then The electrolyte is separated from the copper ore powder to remove lead and zinc from the copper ore powder; in the above method, the molar concentration of NaCl in the electrolytic system is 3.8 mol / L; NaCl+HCl electrolyte system is added to the dispersed Agent C 12 h 25- OSO 3 Na (sodium lauryl sulfate), C 12 h 25- OSO 3 The amount of Na used was 115 g.
[0056] The electrolysis parameters are controlled as follows: the electrolysis temperature is 62°C, t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap