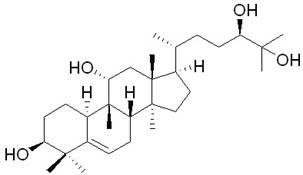
Application of mogrol H9 for preparing antitumor drugs
An anti-tumor drug and tumor technology, applied in the chemical industry, can solve the problems of low content and difficult to separate and purify in large quantities, and achieve the effects of inhibiting proliferation, small side effects, and obvious tumor inhibiting effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 MTS method to determine the growth inhibitory effect of H9 on liver cancer cells
[0041] HepG2 (purchased from ATCC) 3×10 3 Each well was inoculated into a 96-well plate, cultured for 24 hours to allow it to adhere to the wall, and then added H9 (Shanghai Institute of Materia Medica, Chinese Academy of Sciences), with 6 concentration gradients and 3 replicate wells for each concentration. Cells at 37°C, 5% CO 2 After culturing for 72 hours under the same conditions, the culture medium was discarded, and the cell viability was measured with an MTS kit (Promega Company).
[0042]The test method is: wash the cells once with serum-free medium, add the pre-prepared MTS chromogenic solution according to the amount of 100 μl / well (add 2 ml of solution 1 and 100 μl of solution 2 to 10 ml of serum-free medium, mix thoroughly uniform). A well without cells was set as the background well to correct for the background light absorption of the solution. Put the cells ...
Embodiment 2
[0048] Example 2 The growth inhibitory effect of H9 on human pancreatic cancer cells
[0049] Using cck-8 kit (Japan Dojin Chemical Research Institute) detection.
[0050] step:
[0051] 1) The PANC-1 cells (purchased from the Cell Bank of the Chinese Academy of Sciences) were evenly planted in a 96-well plate, and the number of cells per well was 3*10 3 indivual.
[0052] 2) Wait for the wall to adhere, add the drug after overnight, add the drug (the concentrations of H9 are 50, 16.67, 5.56, 1.85, and 0.62 μM), and each concentration has 3 replicate wells.
[0053] 3) After culturing for 48 hours, replace the complete medium with a mixture of serum-free medium and CCK8 (10:1), and incubate in a 37°C incubator for 2 hours.
[0054] 4) Take 450nm as the measurement wavelength and 650nm as the reference wavelength, and measure the reading on a microplate reader.
[0055] Results: The IC50 value of H9 on PANC-1 cells was 283.8 μM.
[0056] The above results indicated that H9...
Embodiment 3
[0057] Example 3 The growth inhibitory effect of H9 on human gastric cancer cells
[0058] The effect of H9 on HGC was tested according to the method in Example 2, and the results showed that the IC50 value of H9 on HGC cells (purchased from ATCC, American Tissue Culture Collection, American Tissue Culture Bank) was 103.69 μM.
[0059] The above results indicated that H9 had a certain inhibitory effect on gastric cancer cell HGC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com