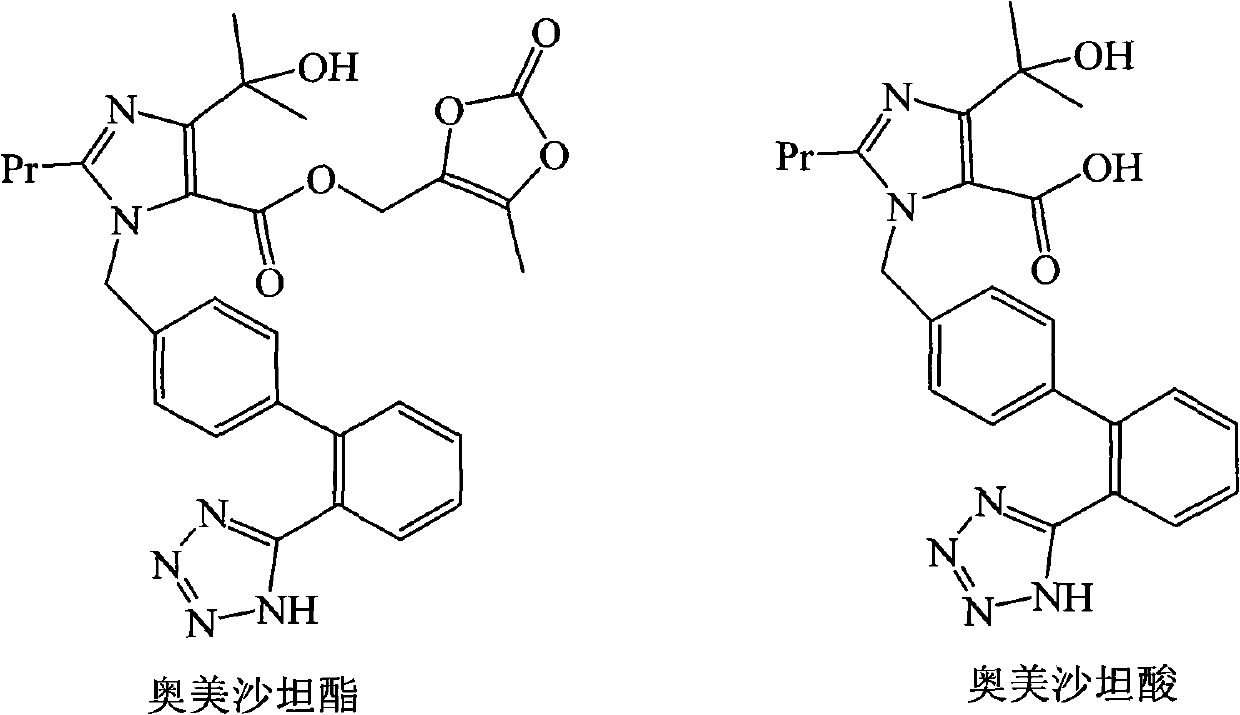
Process for refining olmesartan medoxomil by adopting mixed solution of acetone and water
A technology of olmesartan medoxomil and mixed solution, applied in the field of medicinal chemistry, can solve the problems of high solvent residue and low acetone solvent residue, and achieve the effects of low acetone residue, stable and uniform crystallization, and good refining effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 25.0g olmesartan medoxomil (containing 1.2% olmesartan acid impurity content) and 375ml acetone into a 1L three-necked flask, heat and mechanically stir and reflux to dissolve. Take another 1L three-necked flask and add 375ml purified water, stir and heat to 45~50℃. Add the acetone / water solution of Olmesartan medoxomil before, and keep the temperature for 1 hour after the addition, stir for 3 hours under cooling in an ice-water bath, filter to dryness, and vacuum dry at 45-50℃ to obtain 23.8g of white solid product omesar The yield is 94.0%, the purity is 99.5%, olmesartan acid impurity is 0.12%, other single impurities are less than 0.1%, and acetone remains 710ppm.
Embodiment 2
[0018] Add 15g olmesartan medoxomil (containing 0.8% olmesartan acid content) and 225ml acetone to a 500ml glass bottle, mechanically stir to reflux and dissolve it, cool slightly, and pour it into a 1L glass bottle containing 225ml 40-45℃ water under stirring. Keep the temperature and stir for 1.5 hours, then cool to 15-20°C and stir for 3.5 hours, filter to dryness, and vacuum dry at 50°C for 16 hours to obtain 13.8 g of olmesartan medoxomil with 92.0% yield and 99.8% purity. Olmesartan acid Impurities are 0.05%, other single impurities are less than 0.1%, and acetone remains 1327ppm.
Embodiment 3
[0020] Add 25.0g olmesartan medoxomil (containing 0.8% olmesartan acid), 250ml acetone and 12.5ml purified water into a 1L three-necked flask, heat and mechanically stir to reflux to dissolve, stop heating and stirring. Add another 1L three-necked flask to 237.5ml purified water, and heat to 45-50℃ with mechanical stirring. Add olmesartan medoxomil acetone / aqueous solution, after the addition, keep stirring at this temperature for 1 hour, stir at room temperature for 2 hours, stir under ice-water bath cooling for 2 hours, filter and dry under vacuum at 45-50℃ to obtain 23.8g of white solid The product olmesartan medoxomil has a yield of 95.2%, purity of 99.8%, olmesartan acid impurity 0.02%, other single impurities are less than 0.1%, and acetone residue is 877 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com