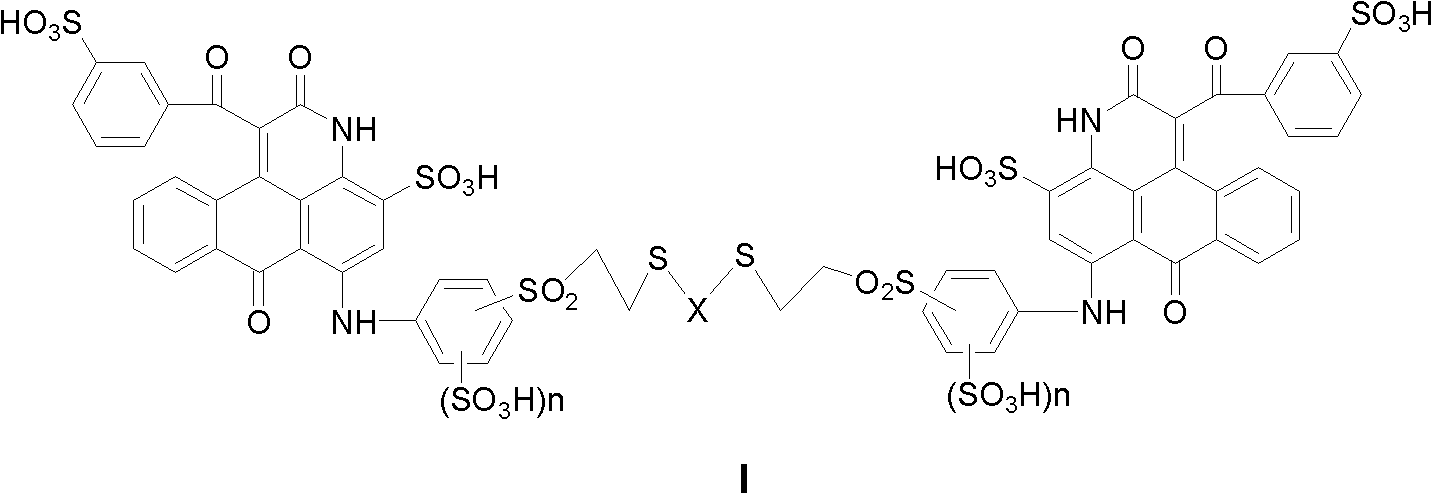
Flexible-chain-contained dikaryotic anthracene pyridine sulfoacid compound or salt thereof, and preparation method and application thereof
A technology for nuclear anthrapyridone sulfonic acid and compound, which is applied in the structural field of dinuclear anthrapyridone sulfonic acid compounds, can solve problems such as unsatisfactory solubility, improve the ability of resisting photooxidation and ozone oxidation, and increase the interaction force , Good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
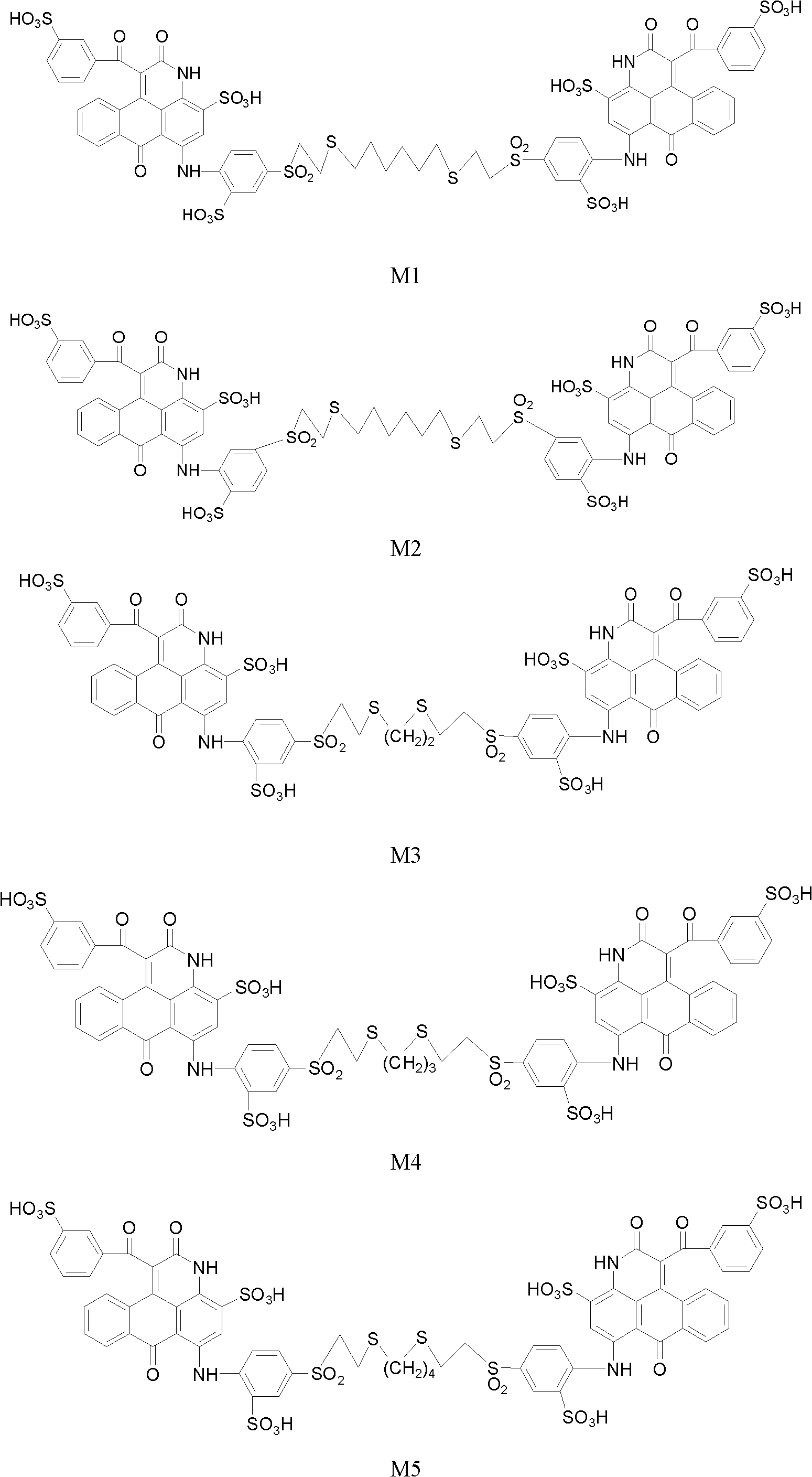
[0085] Example 1. Preparation of compound M1
[0086] (1) Add 125.2 parts of commercial dye (II-1) into 500 parts of water and stir to dissolve, then add 13.5 parts of 1,6-hexanedithiol, namely HS(CH 2 ) 6 SH, stir, gradually raise the temperature to 55-60°C, continuously add 20% sodium hydroxide aqueous solution dropwise to maintain the pH 6-7 until the pH is constant at pH 9, at this time the dye precipitates, filter, and dry to obtain 110 parts of derivative M1 -B. The maximum absorption in water is 603nm, mass spectrum m / z(-): 558.0([ M -2H] 2 - / 2]), 1117.1([ M -H] -1 ), 1139.1 ([ M -2H+Na] -1 ), the intermediate dye product (with M=H free sulfonic acid SO 3 H meter) the most abundant accurate molecular mass M for 1118.1.
[0087]
[0088] (2) In 300 parts of xylene, add 100 parts of dimethyl sulfoxide, while stirring, put in 128 parts of blue dye M1-B, 7.5 parts of sodium carbonate, 180 parts of ethyl benzoyl acetate and heat up. Carry out the reaction at 1...
Embodiment 2
[0092] Embodiment 2. preparation compound M3, M4, M5, M6
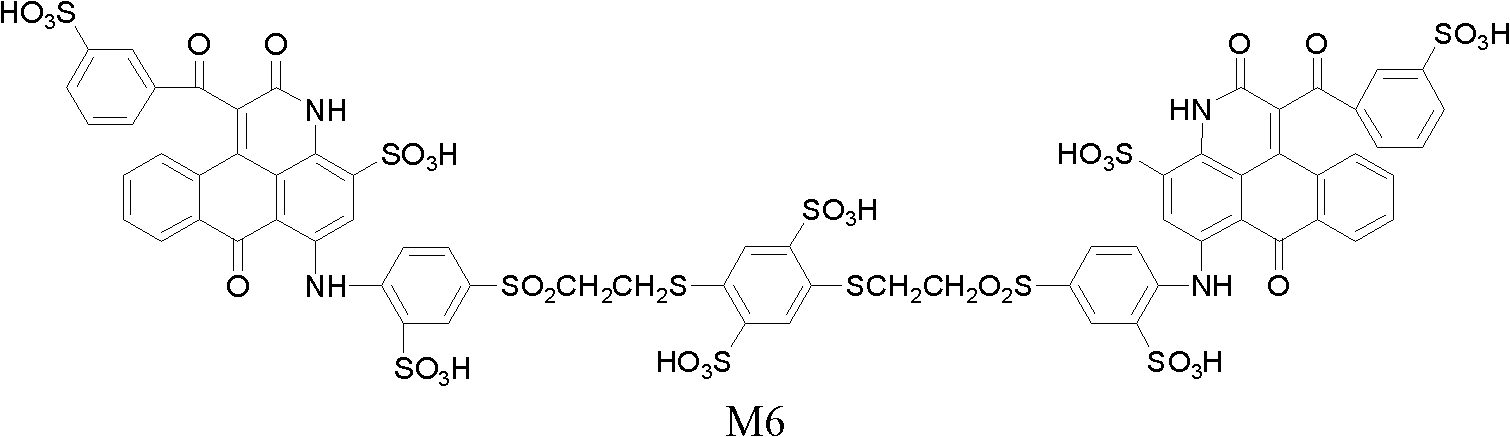
[0093] By the same method as in Example 1, use 1,2-ethanedithiol, 1,3-propanedithiol, 1,4-butanedithiol, 1,4-benzenedithiol respectively, and use different bases The magenta dyes M3, M4, M5 and M6 with the general structural formula (I-1) are prepared by MOH neutralization and MCl salting out. Linker X and sulfonic acid counterion M are shown in Table 1.
[0094]
[0095] Table 1
[0096]
Embodiment 3
[0097] Example 3. Preparation of compound M2
[0098] (1) Add 125.2 parts of C.I. Reactive Blue 19 commercial dye (structural formula II-2) to 500 parts of water and stir to dissolve, then add 13.5 parts of 1,6-hexanedithiol and stir, and gradually heat up to 55-60°C. 20% lithium hydroxide aqueous solution was added dropwise to maintain pH 6-7 until the pH was constant at pH 9, filtered, 10% lithium chloride aqueous solution washed the filter cake, and dried to obtain 112 parts of derivative M2-B. The maximum absorption in water is 603nm, mass spectrum m / z(-): 558.0([ M -2H] 2- / 2]), 1117.1([ M -H] -1 ), 1139.1 ([M -2H+Na] -1 ),. Intermediate dye product M2-B (with free sulfonic acid SO 3 H meter) the most abundant accurate molecular mass number M is 1118.1.
[0099]
[0100] (2) In 300 parts of xylene, add 100 parts of dimethyl sulfoxide, while stirring, put 128 parts of the compound of formula M2-B, 7.5 parts of sodium carbonate, 180 parts of ethyl benzoyl acetate ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap