Method for catalyzing primary amine-acrylate biaddition reaction and application thereof
A technology for acrylates and catalyzing primary amines, used in the preparation of cyanide reactions, chemical instruments and methods, and the preparation of organic compounds, etc. The effect of lithium ion transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
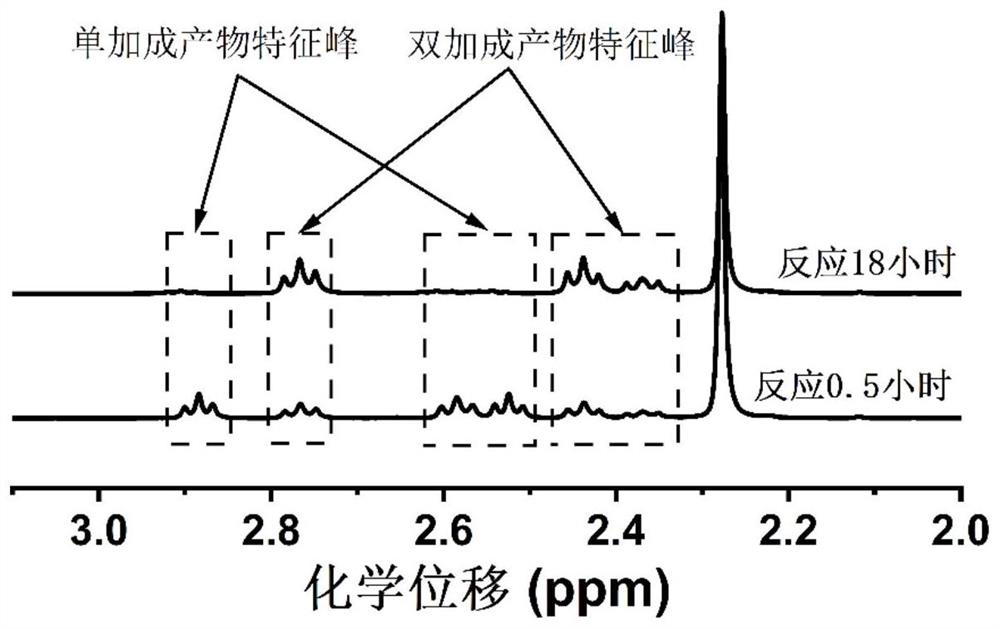
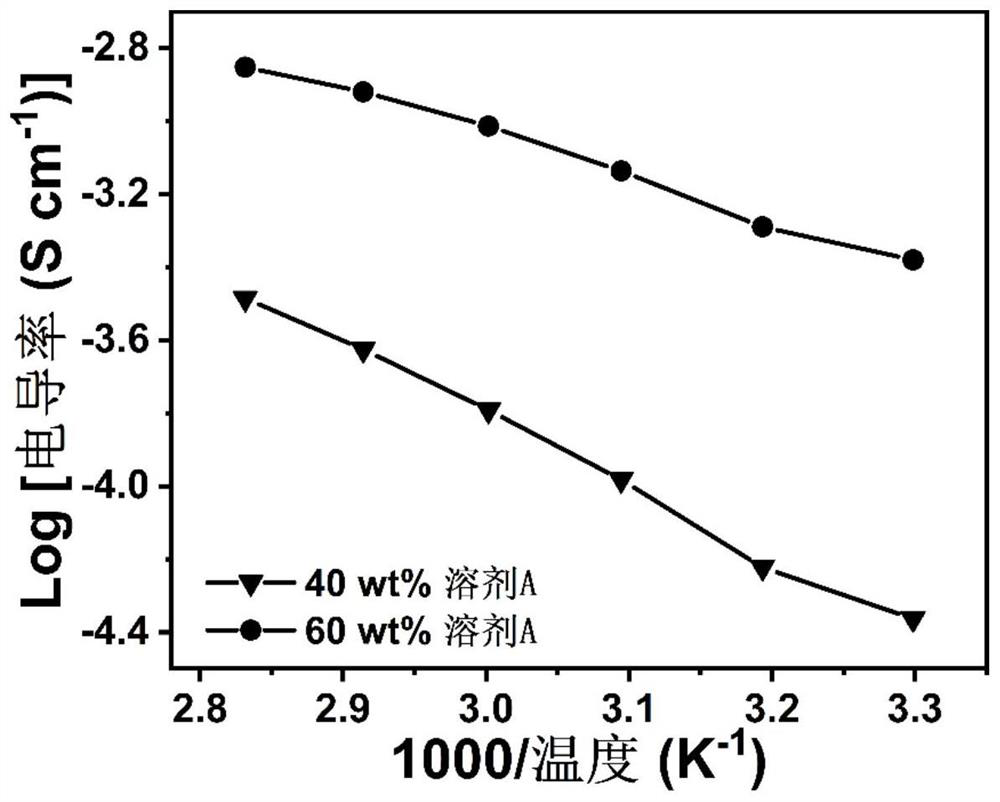
[0032] On the other hand, the present invention also provides an in-situ preparation method of a gel polymer electrolyte, comprising the steps of: mixing a small-molecule organic plasticizer in which a lithium salt is dissolved with a primary amine monomer and an acrylate monomer After stirring and mixing, and standing for a period of time, the primary amine monomer and the acrylate monomer undergo a double addition reaction, and in-situ polymerization is performed to obtain a gel polymer electrolyte. Specifically, the lithium salt and the small molecular organic plasticizer are mixed in a certain proportion, and after stirring evenly, a catalytic solvent is formed; the primary amine monomer and the acrylate monomer are mixed in a certain proportion, and the catalytic solvent is added to form a The precursor liquid, the precursor liquid is allowed to stand for reaction, and the gel polymer electrolyte is obtained by in-situ polymerization.
[0033] In some embodiments, the lit...
Embodiment 1
[0041] A method for a lithium salt-catalyzed primary amine-acrylate double addition reaction, comprising the following steps:
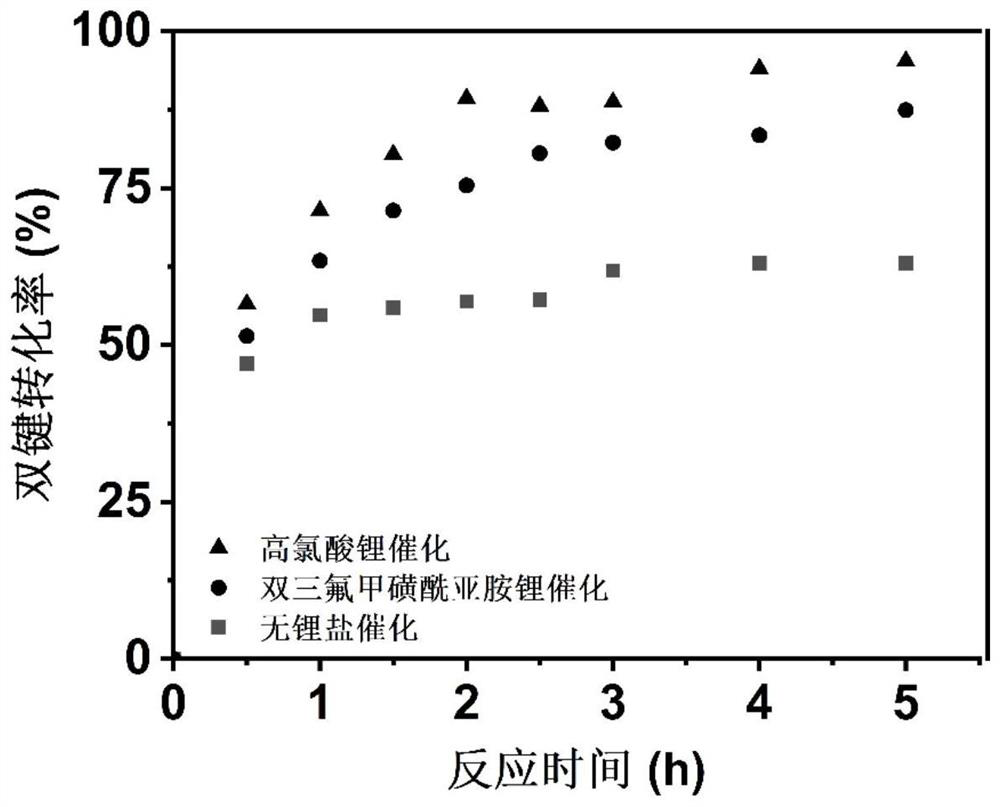
[0042] Mix lithium bistrifluoromethanesulfonimide, mesitylene and methyl acrylate at 1:1:20, and after the lithium salt is completely dissolved, add n-propylamine with a molar ratio of 2:1 to methyl acrylate into the reaction flask , carry out primary amine-acrylate double addition reaction, carry out nuclear magnetic test every half an hour, use mesitylene as calibration, calculate the conversion rate of double bonds. The conversion was 51.43% after half an hour and 87.43% after 5 hours.
Embodiment 2
[0044] A method for a lithium salt-catalyzed primary amine-acrylate double addition reaction, comprising the following steps:
[0045] Mix lithium perchlorate, mesitylene and methyl acrylate at 1:1:15. After the lithium salt is completely dissolved, n-propylamine with a molar ratio of 2:1 to methyl acrylate is added to the reaction flask to carry out primary amine-acrylic acid. For ester double addition reaction, nuclear magnetic test was carried out every half an hour, and the conversion rate of double bond was calculated with mesitylene as the calibration. The conversion was 56.55% after half an hour and 95.24% after 5 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com