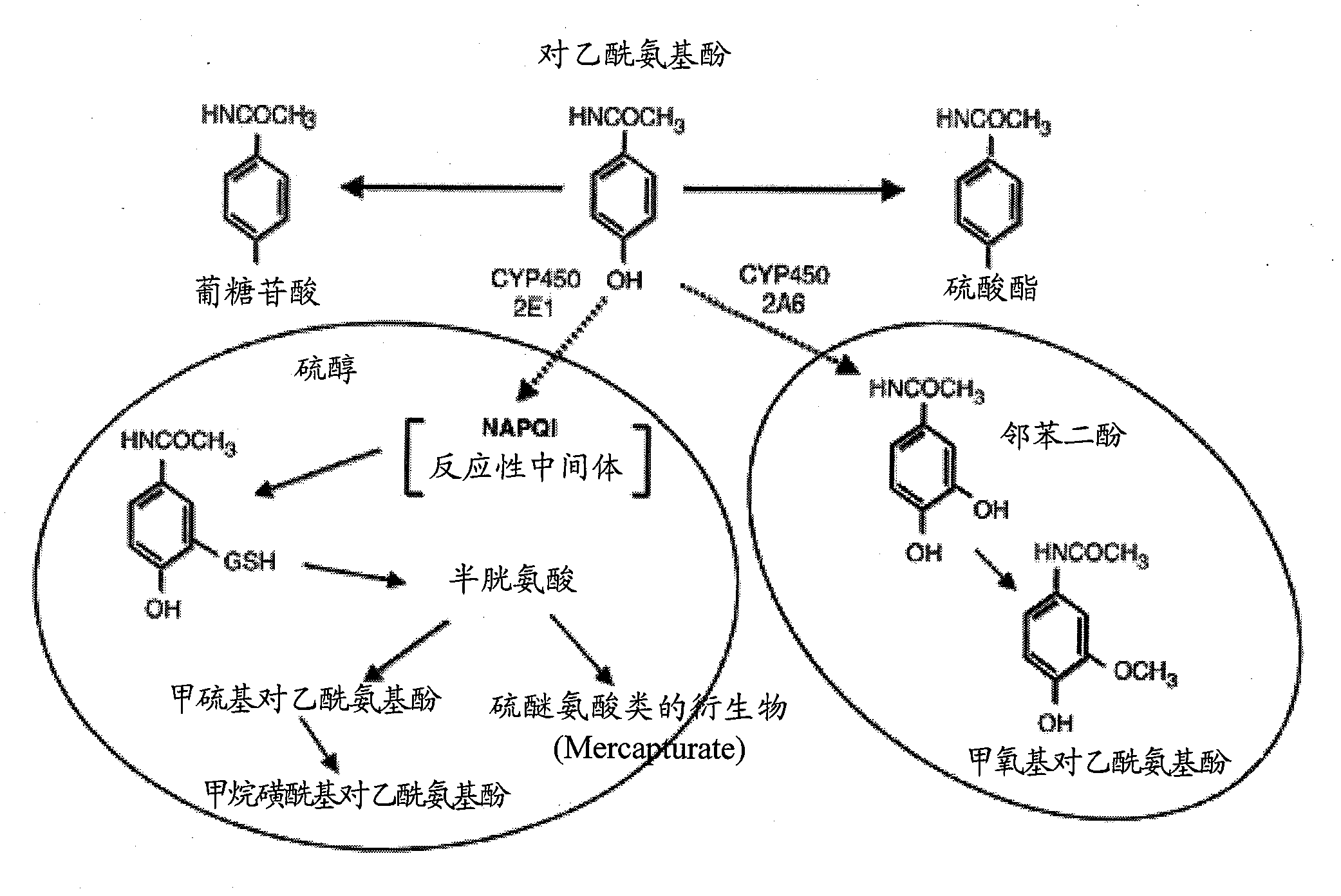
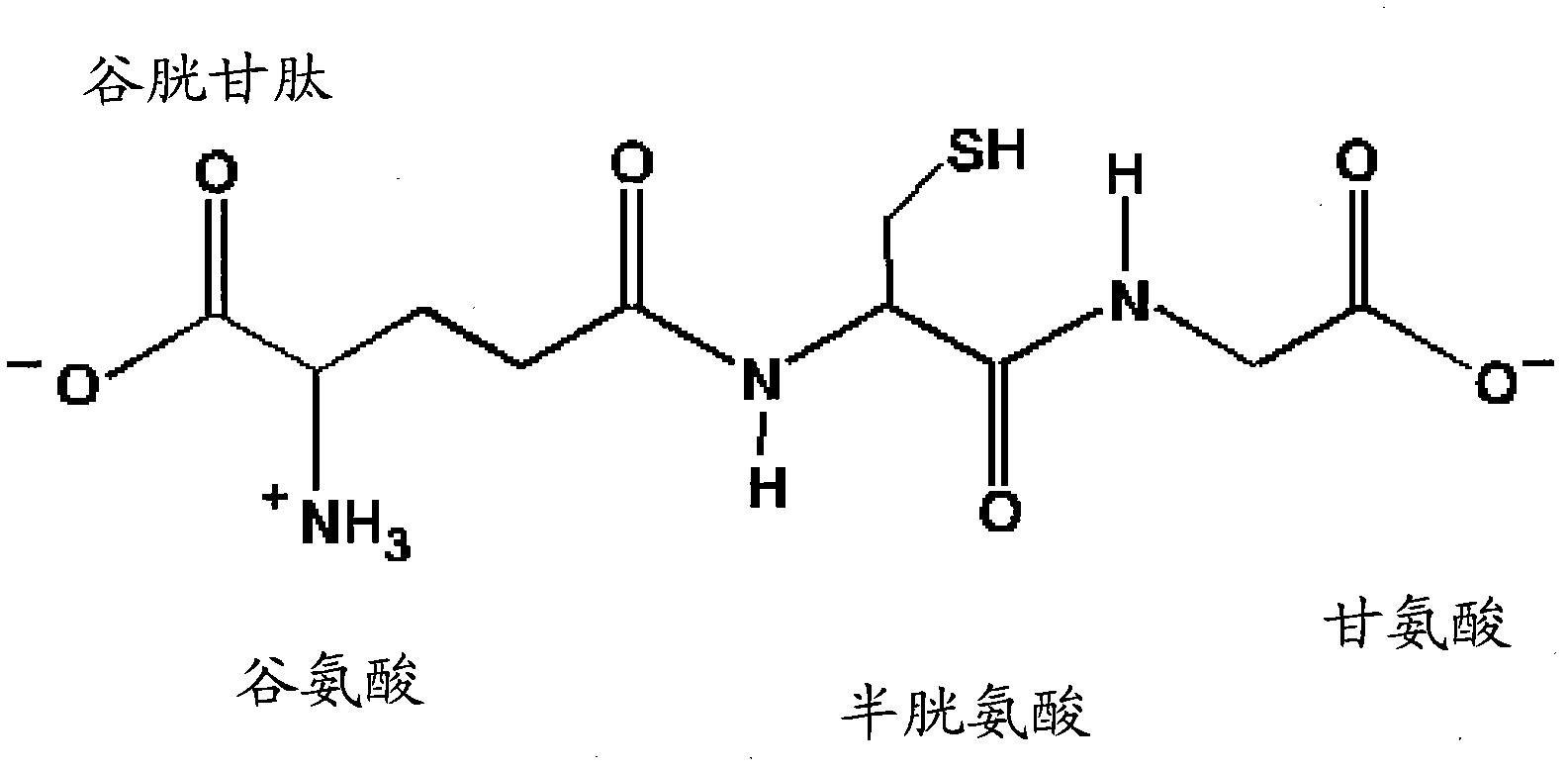
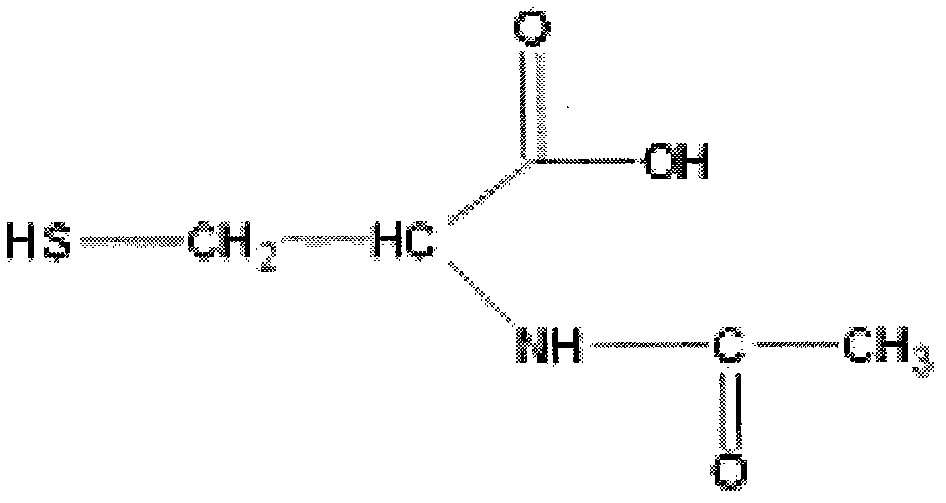
N-acetyl cysteine compositions and methods to improved the therapeutic efficacy of acetaminophen
A paracetamol and acetylcysteine technology, applied in the field of N-acetylcysteine composition and improving the therapeutic efficacy of paracetamol, can solve problems such as allowable dose limitation of treatment plan, limited treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0063] According to one embodiment, the molar ratio of APAP:NAC in the composition of the invention is 1:15 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:10 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:5 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:4 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:3 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:2 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:1 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:0.5 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:0.25 (w / w). According to another embodiment, the molar ratio of APAP:NAC in said composition is 1:0.125 (w / w). Accor...
Embodiment 1
[0157] Example 1. Veterinary use of compositions comprising acetaminophen-N-acetylcysteine
[0158] Veterinarians have experimented with many preparations to provide pain relief in a variety of animal species. Many of these species are more susceptible to the toxic effects of acetaminophen than humans. For example, toxicity was demonstrated in dogs at doses >40 mg / kg compared to doses >140 mg / kg in humans. The most severe toxicity was in felines, where acetaminophen toxicity and death were demonstrated in felines at doses as low as 5-10 mg / kg. This toxicity limits the therapeutic use of acetaminophen in animals.
[0159] According to the present invention, animals needing antipyretic or analgesia are treated with a unit dose of APAP and a therapeutically enhanced amount of NAC so that paracetamol and N-acetylcysteine are contained in the composition of paracetamol and N-acetylcysteine. -The molar ratio of acetylcysteine is in the condition existing composition treatment...
Embodiment 2
[0160] Example 2. Long-term use of an acetaminophen-NAC composition in an adult patient to control osteoarthritis pain
[0161] Adult patients take a standard dose of 1000 mg of acetaminophen four times daily for osteoarthritis pain. The patient obtained the same pain relief by taking 750 mg of acetaminophen-NAC four times a day, and the efficacy of the acetaminophen-NAC formulation did not diminish during long-term use of the acetaminophen-NAC composition.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com