Probiotic derived non-viable material for allergy prevention and treatment
A composition, mixture technology, applied in the field of diet or nutritional products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] In batch fermentation, LGG is grown under physiological conditions. The pH was kept constant at pH 6 by adding 33% NaOH and the temperature was maintained at 37°C. Stirrer speed is 50 rpm, headspace with N 2 rinse. The following medium (modified MRS broth) was provided (Table 1).
[0081] Table 1
[0082]
[0083]
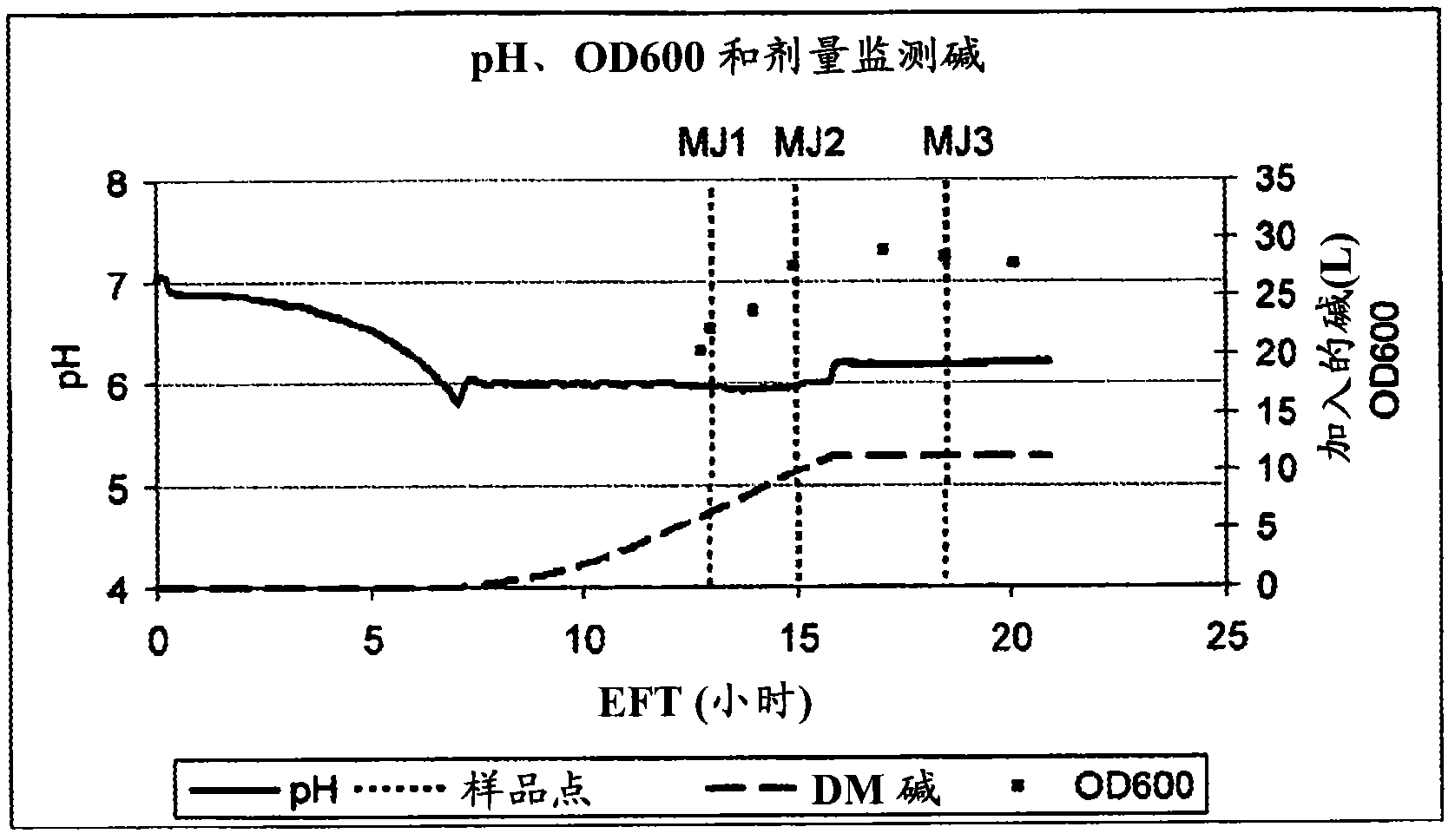
[0084] Bacterial growth is depicted in Figure 1(a) and (b).
[0085] Figure 1a The evolution of pH, amount of titrated NaOH (33%) (DM base = dose monitoring base) and OD600 during the LGG fermentation is shown. Refer to the legend given in the figure. pH and OD600 measurements allow the determination of bacterial growth in the fermenter; where the need to add NaOH to maintain a pH of 6 correlates with lactate production (i.e. a measure of bacterial metabolic activity), OD600 correlates with the number of bacteria in the fermenter Density measurement.
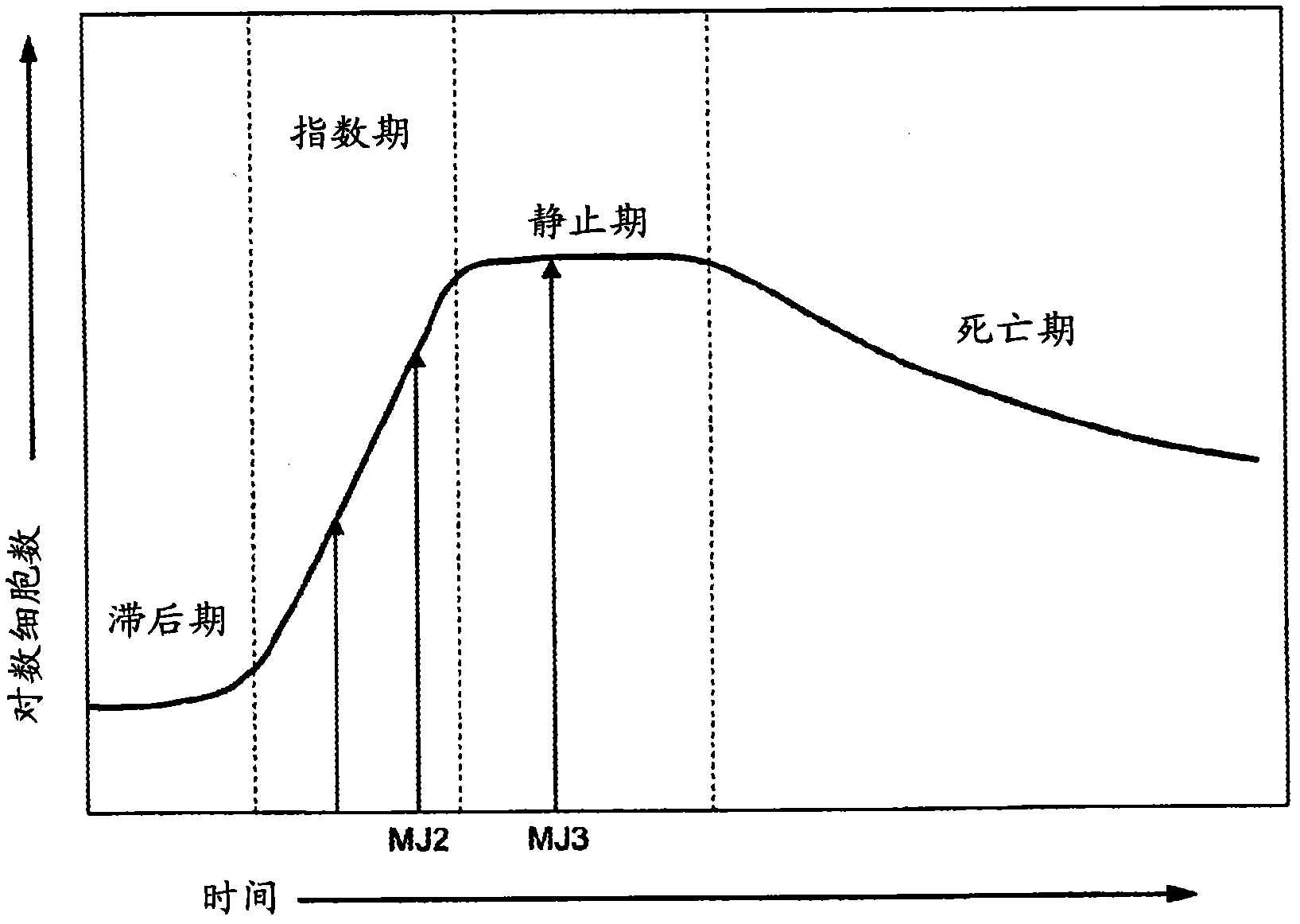
[0086] exist Figure 1b The middle vertical axis represents, on a logarithmic scale, the bac...
Embodiment 2
[0089] Similar to Example 1, LGG culture was carried out on the basis of a modified medium. In the absence of Tween, the oil was added as follows:
[0090] (a) Oleic acid in concentrations of 1 g / kg, 2 g / kg and 4 g / kg.
[0091] (b) Linseed oil In concentrations of 1 g / kg, 2 g / kg and 4 g / kg.
[0092] (c) Olive oil In concentrations of 1 g / kg, 2 g / kg and 4 g / kg.
[0093] (d) Rapeseed oil In concentrations of 1 g / kg, 2 g / kg and 4 g / kg.
[0094] (e) Sunflower oil In concentrations of 1 g / kg, 2 g / kg and 4 g / kg.
[0095] In all of these tests (a)-(e), successful LGG growth was observed, comparable to growth in Tween-containing media. Other than that, it is amazing to be able to grow LGG successfully also without adding Tween or any oil.
Embodiment 3
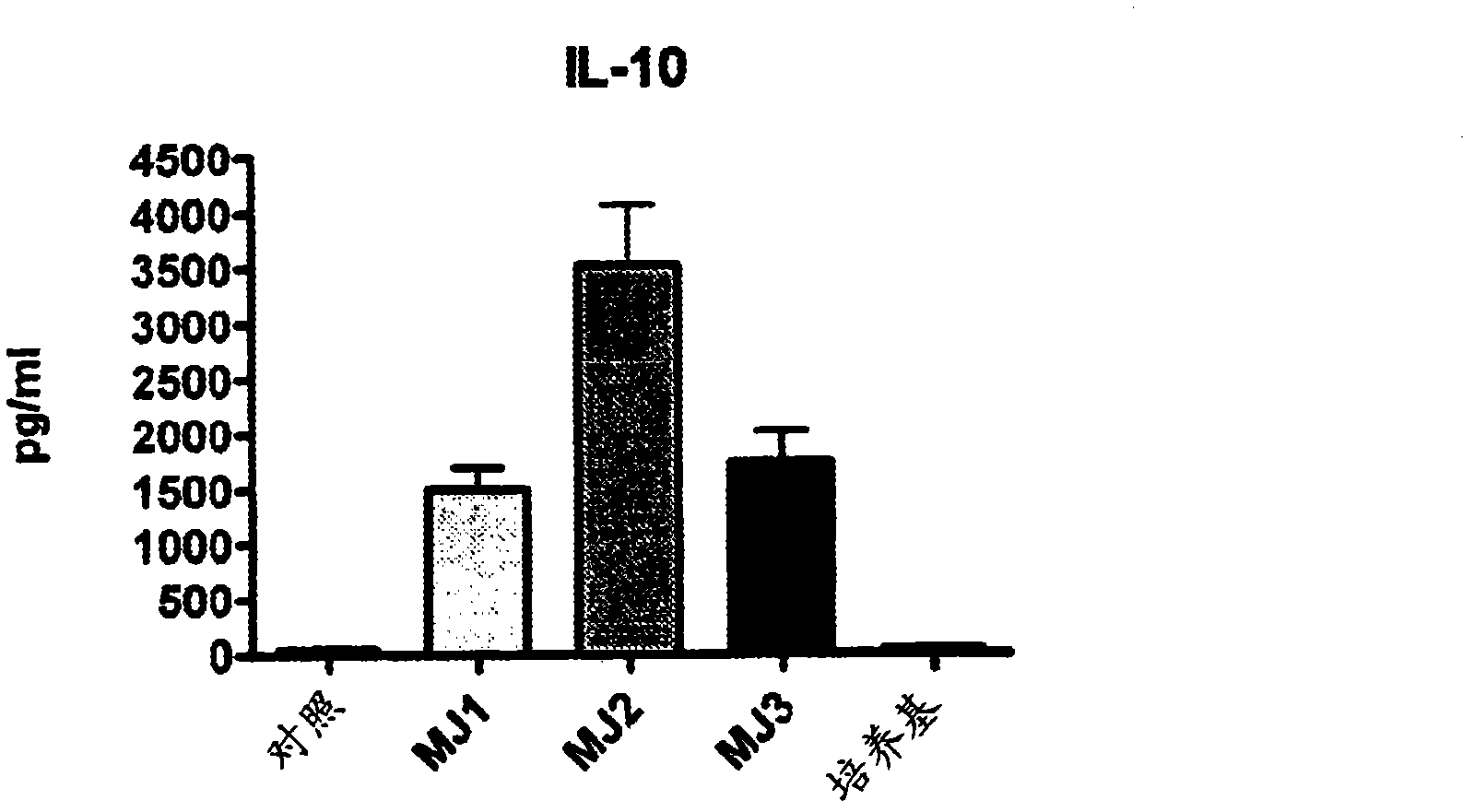
[0097] In this example, the supernatant obtained as in Example 1 was screened for anti-allergic and anti-inflammatory activities using RAW 264 cells (mouse macrophage cell line) in an in vitro model recognized in the art. RAW cell cultures showed substantially increased production of the regulatory cytokine IL-10 during incubation with MJ2 supernatant sample harvests of LGG cultures compared to other supernatant sample harvests. See figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com