Specific clindamycin hydrochloride superfine powdered lyophilized preparation and preparation method thereof
A technology of clindamycin hydrochloride and lincomycin hydrochloride, which is applied in the field of medicine, can solve the problems of large toxic and side effects, small specific surface area, and low clarity, and achieve improved utilization, large specific surface area, and good clarity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
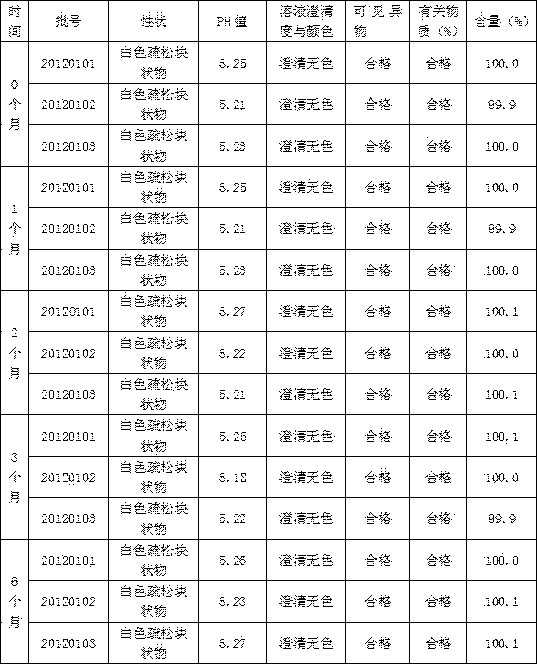
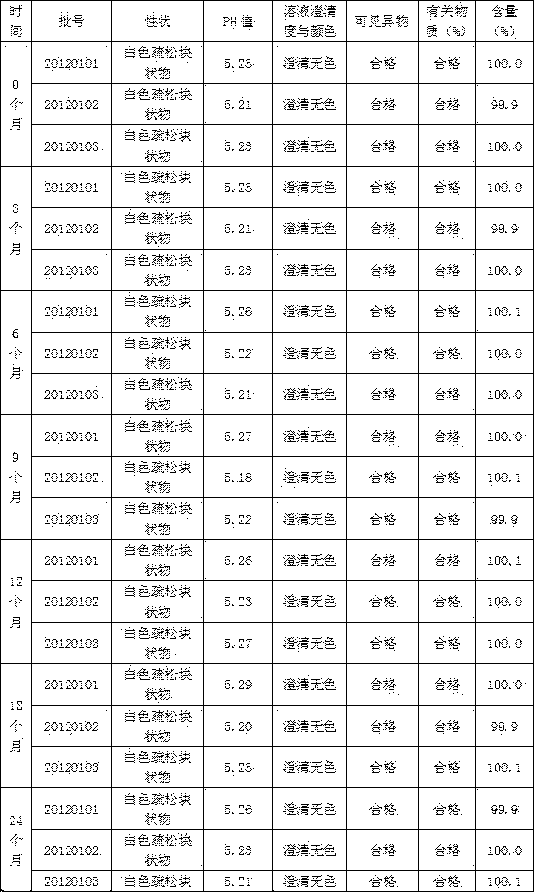
Image
Examples
Embodiment 1
[0025] 80 kg of chloroform, 11 kg of N,N-dimethylformamide (DMF), 10 kg of lincomycin hydrochloride (HPLC purity 99.6%), antioxidant tetrakis[β-(3,5-di 50 grams of tert-butyl-4-hydroxyphenyl) propionic acid] pentaerythritol ester was added to the reaction kettle, and then 12 kilograms of solid phosgene was added in batches, and at 40 ° C, the reaction was incubated for 24 hours, and the hydrochloric acid in the reaction system was detected by HPLC. The content of lincomycin is less than 1.0%, and after the reaction is completed, the temperature of the reaction solution is lowered to 0°C.
[0026] Add 40 kg of purified water and 40 L of 20% sodium hydroxide aqueous solution to the reaction solution, heat up, hydrolyze at 35° C., leave to stand for stratification, and extract the organic phase twice with 40 kg of chloroform for the aqueous phase. Combine the organic phases, wash them with water, concentrate the organic phases under reduced pressure at 55°C to get chloroform, add...
Embodiment 2
[0033] In turn, 90 kg of chloroform, 13 kg of N,N-dimethylformamide (DMF), 10 kg of lincomycin hydrochloride (HPLC purity 99.6%), and the antioxidant tetrakis[β-(3,5-di 55 grams of tert-butyl-4-hydroxyphenyl)propionic acid]pentaerythritol ester was added to the reaction kettle, and then 13 kilograms of solid phosgene was added in batches, and at 45° C., the insulation reaction was carried out for 22 hours, and the hydrochloric acid in the reaction system was detected by HPLC. The content of lincomycin is less than 1.0%, and after the reaction is completed, the temperature of the reaction solution is lowered to 2°C.
[0034]Add 50 kg of purified water and 30 L of aqueous sodium hydroxide solution with a mass concentration of 25% to the reaction solution, raise the temperature, perform hydrolysis at 35°C, leave to stand and separate the layers, and extract the organic layer twice with 40 kg of chloroform in the aqueous phase , combine the organic phases, wash with water, concent...
Embodiment 3
[0041] 100 kg of chloroform, 15 kg of N,N-dimethylformamide (DMF), 10 kg of lincomycin hydrochloride (HPLC purity 99.6%), antioxidant tetrakis[β-(3,5-di 60 grams of tert-butyl-4-hydroxyphenyl) propionate] pentaerythritol ester was added to the reaction kettle, and then 14 kg of solid phosgene was added in batches, and at 50 ° C, the reaction was incubated for 18 hours, and the hydrochloric acid in the reaction system was detected by HPLC. The content of lincomycin is less than 1.0%, and after the reaction is completed, the temperature of the reaction solution is lowered to 5°C.
[0042] Add 60 kg of purified water and 25 L of aqueous sodium hydroxide solution with a mass concentration of 30% to the reaction solution, raise the temperature, perform hydrolysis at 35°C, stand and separate the layers, and extract the organic layer twice with 40 kg of chloroform in the aqueous phase , combine the organic phases, wash with water, concentrate the organic phase under reduced pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com