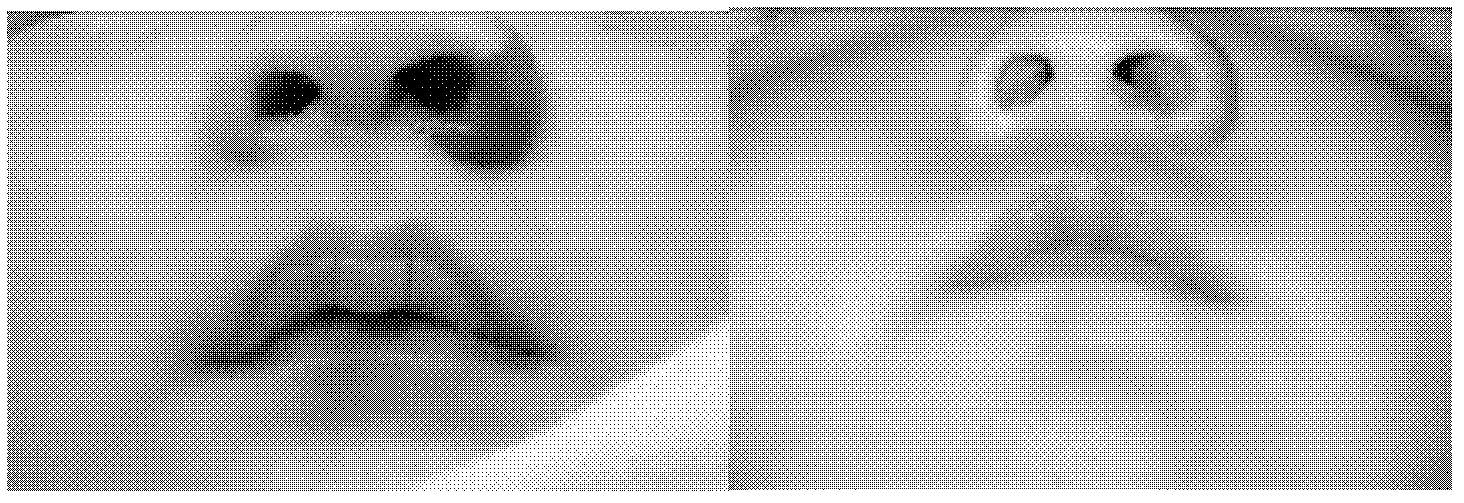
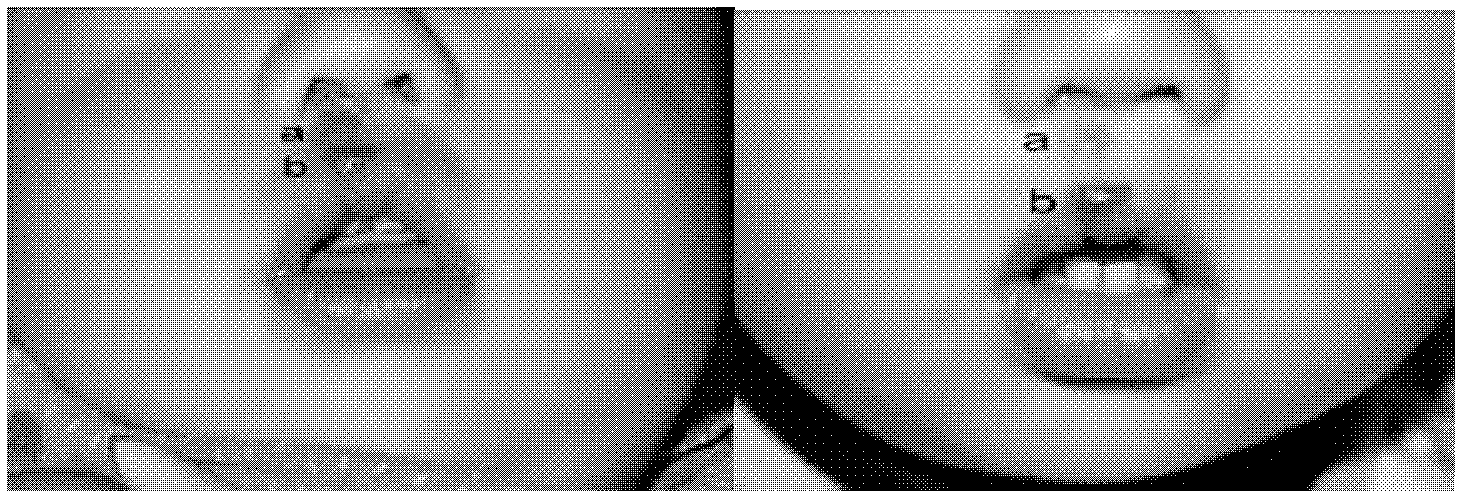
External medicament for treating infant hemangioma and preparation method thereof
A technology for infantile hemangioma and topical drugs, applied in the field of medicine, can solve problems such as not recommended for use in children, unclear side effects of drugs, and limitations in clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Ratio: 10 parts of propranolol, 50 parts of stearic acid, 40 parts of glyceryl monostearate, 50 parts of white petrolatum, 5 parts of laurozone, 5 parts of oleic acid, 10 parts of Tween-80, 70 parts of glycerin Parts, 500 parts of deionized water, 1 part of ethyl paraben.
[0033] Its preparation method is:
[0034] According to the solubility properties of the pharmaceutical composition, it is divided into the following two parts
[0035] Oily components: stearic acid, glyceryl monostearate, white petrolatum, laurocapram, oleic acid
[0036] Water-soluble components: Tween-80, glycerin, deionized water, propranolol, ethyl paraben
[0037] Accurately weigh the oily component and the water-soluble component respectively, heat them separately and keep them at 75°C±2°C; then slowly add the oily component into the water-soluble component, and keep stirring in the same direction until the emulsification is complete and condense into a paste.
[0038] Quality Control
[...
Embodiment 2
[0042] Proportion: 50 parts of propranolol, 220 parts of stearic acid, 150 parts of glyceryl monostearate, 200 parts of white petrolatum, 40 parts of laurozone, 40 parts of oleic acid, 60 parts of Tween-80, 300 parts of glycerin Parts, 1000 parts of deionized water, 10 parts of ethyl paraben.
[0043] Its preparation method is:
[0044] According to the nature of the pharmaceutical composition, it is divided into the following two parts
[0045]Oily components: stearic acid, glyceryl monostearate, white petrolatum, laurocapram, oleic acid
[0046] Water-soluble components: Tween-80, glycerin, deionized water, propranolol, ethyl paraben
[0047] Accurately weigh the oily component and the water-soluble component respectively, heat them separately and keep them at 75°C±2°C; then slowly add the oily component into the water-soluble component, and keep stirring in the same direction until the emulsification is complete and condense into a paste.
Embodiment 3
[0049] Proportion: 20 parts of propranolol, 100 parts of stearic acid, 80 parts of glyceryl monostearate, 100 parts of white petrolatum, 15 parts of laurozone, 15 parts of oleic acid, 30 parts of Tween-80, 120 parts of glycerin Parts, 700 parts of deionized water, 3 parts of ethyl paraben.
[0050] Its preparation method is:
[0051] According to the nature of the pharmaceutical composition, it is divided into the following two parts
[0052] Oily components: stearic acid, glyceryl monostearate, white petrolatum, laurocapram, oleic acid
[0053] Water-soluble components: Tween-80, glycerin, deionized water, propranolol, ethyl paraben
[0054] Accurately weigh the oily component and the water-soluble component respectively, heat them separately and keep them at 75°C±2°C; then slowly add the oily component into the water-soluble component, and keep stirring in the same direction until the emulsification is complete and condense into a paste.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com