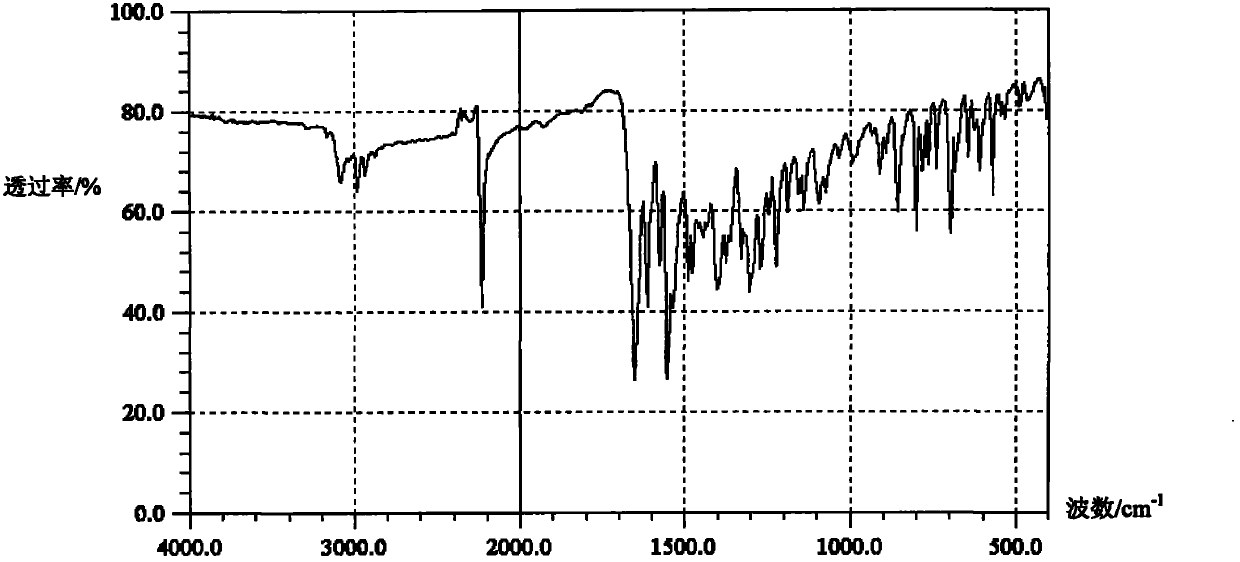
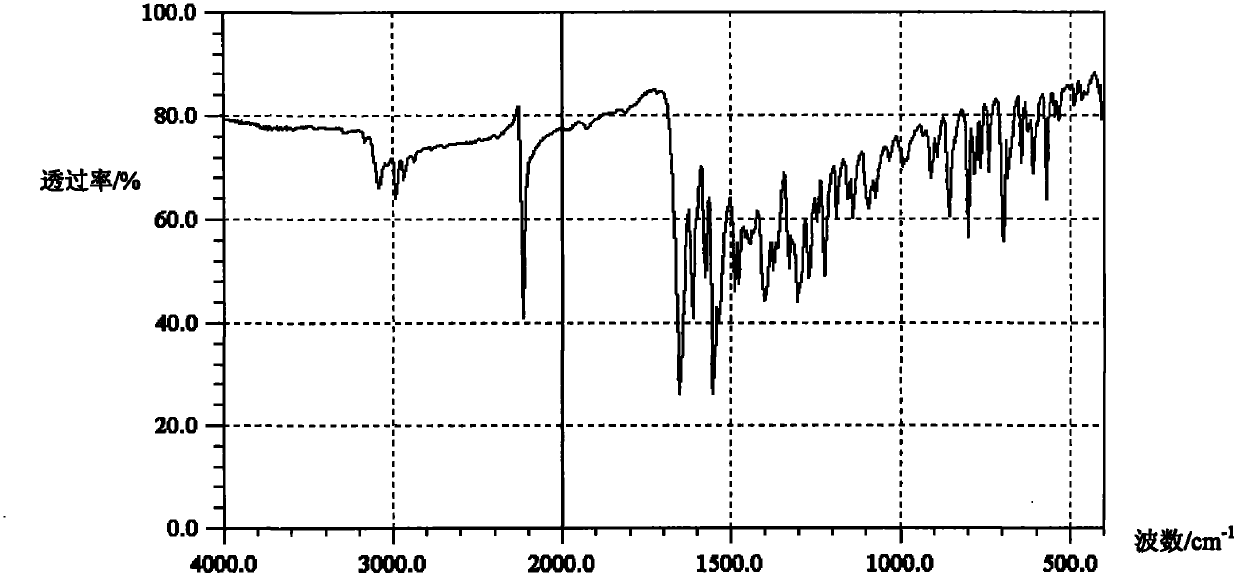
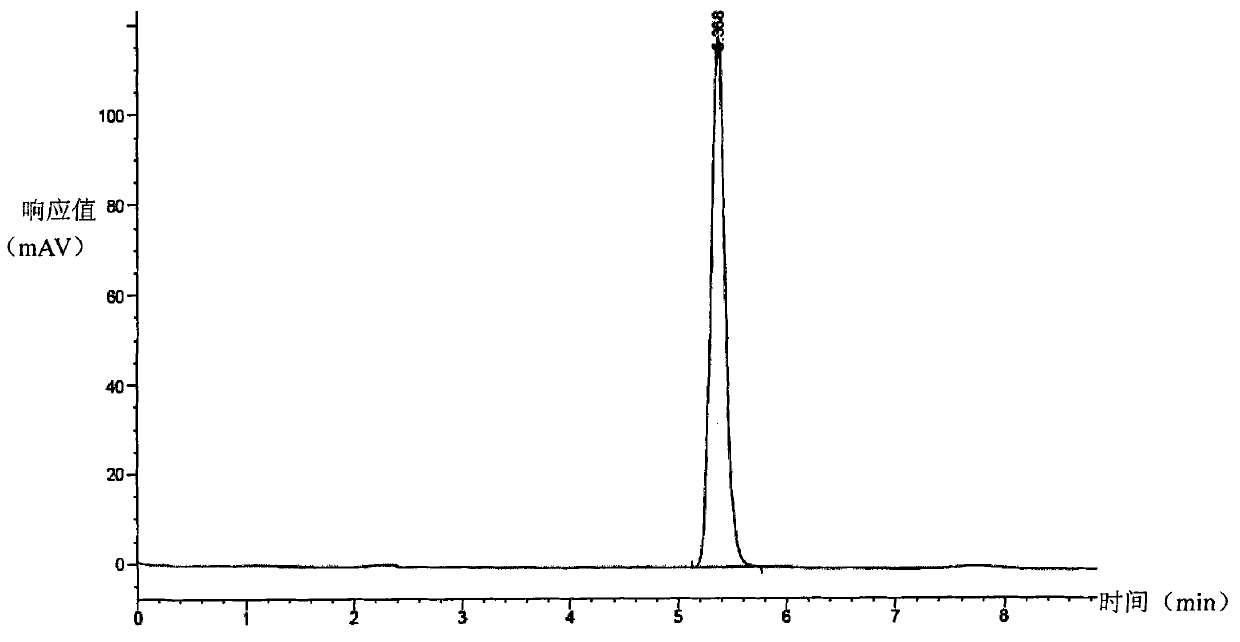
Preparation method for zaleplon
A technology of zaleplon and aminoacetophenone, which is applied in the field of preparation of zaleplon, can solve the problems of difficult mass production, large amount of dimethyl acetal, cumbersome operation, etc., and achieve low production cost and high yield , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1, the preparation of 3-(N-acetyl) aminoacetophenone:
[0041] Add 162.19g of 3-aminoacetophenone and 135.11g of acetic acid into the flask and stir, add 144.97g of acetic anhydride, the reaction generates heat, and the solid dissolves. After stirring for 50 minutes, place it at 0°C for 4 hours, add about 600ml of water to wash the product, and filter , and the solid was vacuum-dried at 50° C. to obtain 192.9 g of a white solid product 3-(N-acetyl)aminoacetophenone, with a yield of 91%.
[0042] 2. Preparation of 3-(N-ethyl-N-acetylamino)acetophenone:
[0043] Add 192.9g of 3-(N-acetyl)aminoacetophenone and 2340ml of acetone into the bottle and stir, then add 347.8g of ethyl iodide, adjust the pH value to 7.5 with 55% KOH aqueous solution, heat and reflux for 3.5 hours, evaporate to Solvent, add 1170ml water to dissolve the product. Use 1170ml dichloromethane to extract the aqueous solution three times, the extracts are combined, and the dichloromethane is evaporated ...
Embodiment 2
[0051] 1, the preparation of 3-(N-acetyl) aminoacetophenone:
[0052] Add 189.22g of 3-aminoacetophenone and 135.11g of acetic acid into the flask and stir, add 134.76g of acetic anhydride, the reaction generates heat, and the solid dissolves. After stirring for 45 minutes, place it at 3°C for 5 hours, add about 600ml of water to wash the product, and filter , the solid was vacuum-dried at 45° C. to obtain 223.72 g of a white solid product, 3-(N-acetyl)aminoacetophenone, with a yield of 90%.
[0053] 2. Preparation of 3-(N-ethyl-N-acetylamino)acetophenone:
[0054] Add 223.72g of 3-(N-acetyl)aminoacetophenone and 2330ml of acetone into the bottle and stir, then add 393.04g of ethyl iodide, adjust the pH value to 8 with 60% KOH aqueous solution, heat and reflux for 4 hours, evaporate to Solvent, add 1200ml water to dissolve the product. Use 1200ml dichloromethane to extract the aqueous solution three times, the extracts are combined, and the dichloromethane is evaporated un...
Embodiment 3
[0062] 1, the preparation of 3-(N-acetyl) aminoacetophenone:
[0063] Add 175.71g of 3-aminoacetophenone and 138.72g of acetic acid into the flask and stir, add 140.88g of acetic anhydride, the reaction generates heat, and the solid dissolves. After stirring for 55 minutes, place it at 3°C for 3 hours, add about 600ml of water to wash the product, and filter , and the solid was vacuum-dried at 45° C. to obtain 211.93 g of a white solid product, 3-(N-acetyl)aminoacetophenone, with a yield of 92%.
[0064] 2. Preparation of 3-(N-ethyl-N-acetylamino)acetophenone:
[0065] Add 211.93g of 3-(N-acetyl)aminoacetophenone and 2350ml of acetone into the bottle and stir, then add 379.63g of ethyl iodide, adjust the pH value to 7 with 55% KOH aqueous solution, heat and reflux for 4 hours, evaporate to Solvent, add 1170ml water to dissolve the product. The aqueous solution was extracted three times with 1170ml of dichloromethane, the extracts were combined, and the dichloromethane was ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap