Noninvasive chronic myelogenous leukemia susceptibility gene assay kit
A myeloid leukemia and detection kit technology, applied in the field of molecular biology, can solve problems such as reduction and loss of body metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1. Use of detection kits
[0021] 1. Extract DNA template
[0022] The epithelial cells of the oral mucosa of the subjects were scraped, and the genomic DNA was extracted by the phenol-chloroform method.
[0023] 2. PCR amplification reaction
[0024] Use the PCR reaction component in the detection kit, which contains the following primer pairs:
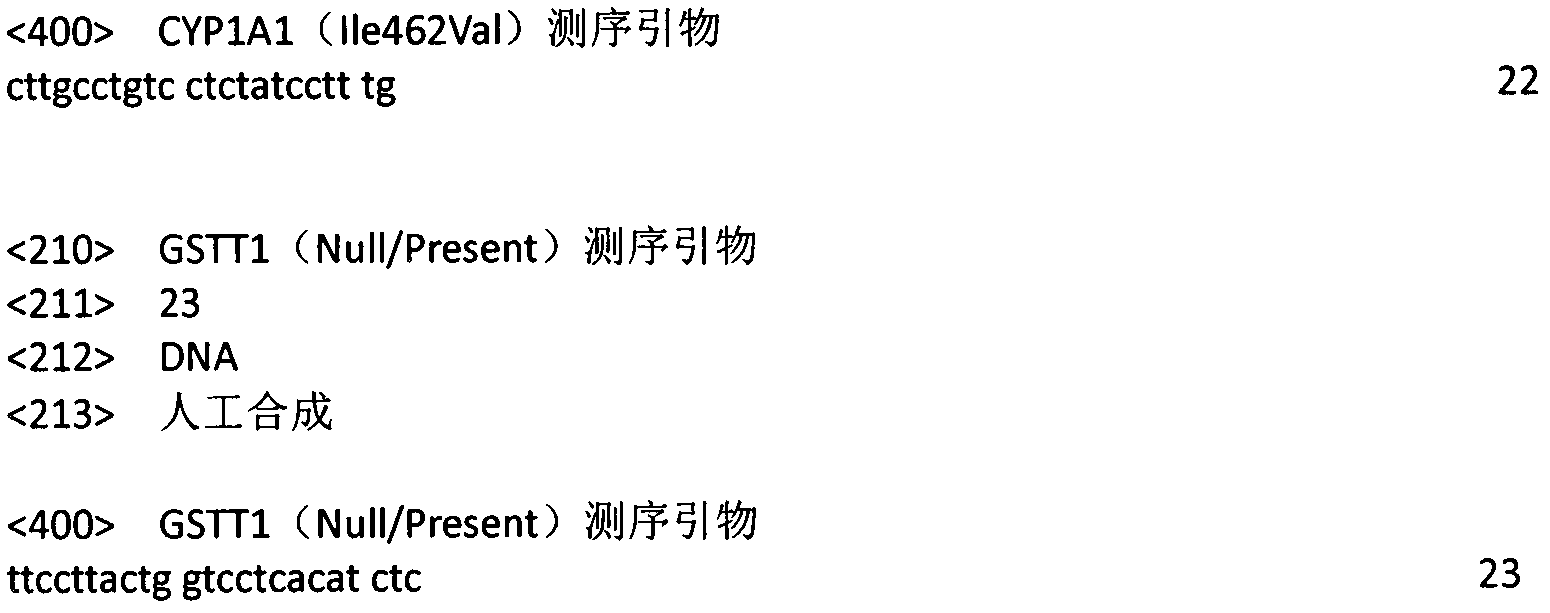
[0025] (1) CYP1A1 (Ile462Val) forward primer: 5'CTTGCCTGTCCTTCTATCCTTTG3'
[0026] CYP1A1 (Ile462Val) reverse primer: 5'CAGGCTGAACCTTAGACCCACA3'
[0027] (2) GSTT1 (Null / Present) forward primer: 5′TTCCTTACTGGTCCTCACATCTC3′
[0028] GSTT1 (Null / Present) reverse primer: 5'TCACCGGATCATGGCCAGCA3'
[0029] The reaction system for PCR amplification is: 10×PCR reaction buffer 2.5μl; 25mM dNTP mixture 0.2μl, 5U / ul Taq enzyme 0.125μl, DNA template 1μl (about 12-15ng), 20uM forward primer and reverse primer Each 0.25μl, ddH2O 19.175μl;
[0030] The reaction conditions are: denaturation and enzyme activation at 94°C ...
Embodiment 2
[0043] Example 2. The service of non-invasive gene detection for preventing the onset of chronic myelogenous leukemia
[0044] 1. Sampling and DNA extraction
[0045] The physicians in the laboratory department of the hospital will guide the subjects to use oral swabs to sample oral epithelial cells, and use the phenol-chloroform method to extract DNA from oral epithelial cells
[0046] 2. Genotype detection
[0047] Using the kit provided by the invention, the ILe462Val site on the CYP1A1 gene of the subject's genomic DNA, whether the 2 single nucleotide polymorphism sites of the GSTT1 gene are missing (Null / Present) carry out DNA sequencing respectively, and determine these Genotypes of 2 SNPs loci.
[0048] 3. Risk assessment of chronic myelogenous leukemia high-risk groups
[0049] Through the analysis of the SNPs genotypes of the subjects, a risk assessment and analysis report of chronic myelogenous leukemia susceptibility genes is issued. The report details the ILe462V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com