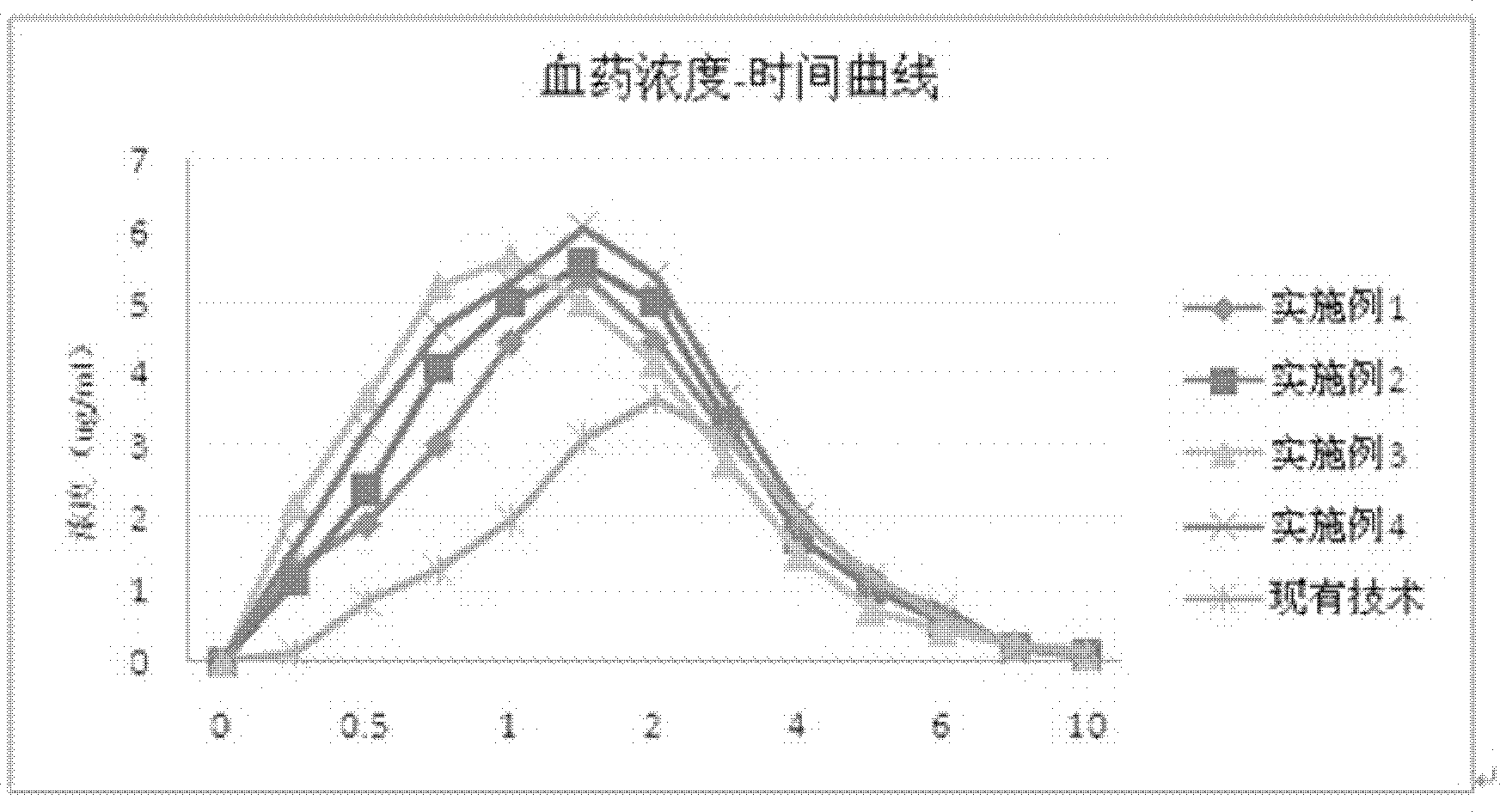Cefuroxime axetil granules and preparation method
A technology of cefuroxime axetil and granules, which is applied in the direction of pharmaceutical formulas, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as poor dispersion and residual bitterness of preparations, and achieve the promotion of drug dissolution, Effect of improving dissolution rate and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A cefuroxime axetil granule comprises the following components by weight percentage:
[0035] Table 1. Embodiment a group of distribution ratio
[0036] components
Weight (kg)
cefuroxime axetil
6.0
Kollidon VA64
6.0
lactose
6.0
Croscarmellose Sodium
1.0
Sucralose
0.8
lemon yellow lozenges
0.005
Sodium carboxymethyl cellulose
0.015
Sweet Orange Flavor
0.18
[0037] A preparation method of cefuroxime axetil granules, comprising the following steps (each component consumption is as described in table 1):
[0038] (1) Mix cefuroxime axetil and Kollidon VA64, add it to a hot-melt extruder, heat and melt at 130°C, and the torque is 5-10N cm (torque: in physics, it is the size of the moment, equal to the force and the arm of force The product of torque is the power to make the object rotate, which in this embodiment refers to the power required to make...
Embodiment 2
[0043] A cefuroxime axetil granule comprises the following components by weight percentage:
[0044] Table 2. Embodiment two groups distribution ratio
[0045] components
Weight (kg)
cefuroxime axetil
3.0
Kollidon VA64
9.0
sucrose
6.3
[0046] Low-substituted hydroxypropyl cellulose
1.2
Red No. 33
0.004
Polyvinylpyrrolidone K30
0.4
strawberry flavor
0.096
[0047] A preparation method of cefuroxime axetil granules, comprising the following steps (each component consumption is as described in table 2):
[0048] (1) Take cefuroxime axetil and Kollidon VA64, add them to a hot-melt extruder, heat and melt at 150°C, and the torque is 5-10N cm (torque: in physics, it is the magnitude of the moment, equal to the force and the force arm Product, torque is the power that makes the object rotate, and in this embodiment refers to the power needed to make the screw of ...
Embodiment 3
[0053] A cefuroxime axetil granule comprises the following components by weight percentage:
[0054] Table 3. Embodiment three groups distribution ratio
[0055] components
Weight (kg)
cefuroxime axetil
1.5
soluplus
7.5
Mannitol
10.2
Crospovidone
0.6
aspartame
0.05
red number 3
0.01
Methylcellulose
0.04
[0056] cherry flavor
0.1
[0057] A preparation method of cefuroxime axetil granules, comprising the following steps (each component consumption is as described in table 3):
[0058] (1) Take cefuroxime axetil and soluplus, put them into a hot-melt extruder, heat and melt at 160°C, and the torque is 5-10N·CM (torque: in physics, it is the magnitude of the moment, which is equal to the product of the force and the arm , Torque is the power that makes object rotate, and in the present embodiment refers to the power needed to make the hot-melt ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap