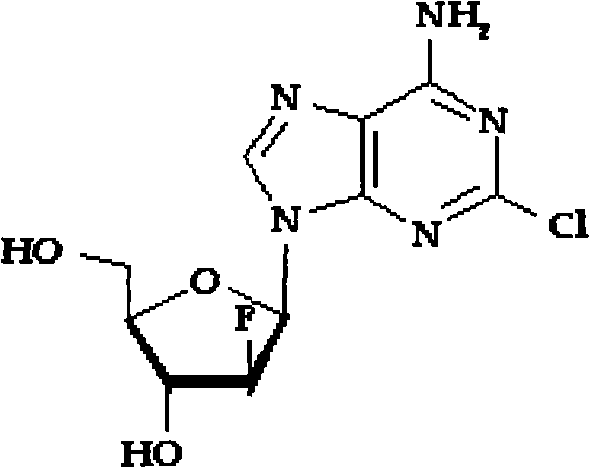
Method for separating and purifying Clofarabine
A technology for separation and purification of clofarabine, applied in the field of separation and purification of crude clofarabine, can solve the problems of large amount of solvent, large pollution, low purity of clofarabine and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Dissolving and filtering:
[0027] Get clofarabine crude product 3.75g, be dissolved in 750mL of boiling aqueous solution, solid clofarabine crude product is dissolved, after dissolving, filter with the organic microporous membrane of 0.45 μm, obtain the solution of 5mg / mL, prepare clofarabine as the next step raw material.
[0028] (2) separation and purification:
[0029] Inject 18 mL of the solution diluted in step (1) into a reversed-phase high-efficiency preparative liquid chromatograph, the mobile phase is composed of acetonitrile-water, the volume ratio of acetonitrile-water is 1:8.8, isocratic elution, flow rate 80mL / min , UV detector online detection, detection wavelength 254nm. The preparation components of clofarabine are collected in a targeted manner to obtain a clofarabine solution.
[0030] (3) Product recycling
[0031] Acetonitrile and water in the clofarabine solution obtained by step (2) high-efficiency preparative liquid chromatography separa...
Embodiment 2
[0033] (1) Dissolving and filtering:
[0034] Take 3.75g of crude clofarabine, dissolve it in 577mL of boiling aqueous solution, dissolve the crude solid clofarabine, and filter it with a 0.45μm organic microporous membrane to obtain a 6.5mg / mL solution, which is used as the next step to prepare clofarabine raw materials.
[0035] (2) separation and purification:
[0036] Inject 15 mL of the solution diluted in step (1) into a reversed-phase high-efficiency preparative liquid chromatograph, the mobile phase is composed of acetonitrile-water, the volume ratio of acetonitrile-water is 1:9, isocratic elution, flow rate 85mL / min , UV detector online detection, detection wavelength 254nm. The preparation components of clofarabine are collected in a targeted manner to obtain a clofarabine solution.
[0037] (3) Product recycling
[0038] Acetonitrile and water in the clofarabine solution obtained by step (2) high-efficiency preparative liquid chromatography separation are remove...
Embodiment 3
[0040] (1) Dissolving and filtering
[0041] Take 3.75g of the crude product of clofarabine, dissolve it in 469mL of boiling aqueous solution, dissolve the crude product of solid clofarabine, and filter it with an organic microporous membrane of 0.45 μm after dissolving to obtain a solution of 8 mg / mL, which is used as the next step to prepare clofarabine raw material.
[0042] (2) Separation and purification
[0043] Inject 18 mL of the solution diluted in step (1) into a reversed-phase high-efficiency preparative liquid chromatograph, the mobile phase is composed of acetonitrile-water, the volume ratio of acetonitrile-water is 1:9.2, isocratic elution, flow rate 90mL / min , UV detector online detection, detection wavelength 254nm. The preparation components of clofarabine are collected in a targeted manner to obtain a clofarabine solution.
[0044] (3) Product recycling
[0045] Acetonitrile and water in the clofarabine solution obtained by step (2) separated by high-effi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com