Pharmaceutically useful heterocycle-substituted lactams
A pharmacy and prodrug technology, which can be used in medical preparations containing active ingredients, antipyretics, drug combinations, etc., and can solve problems such as poor clinical effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
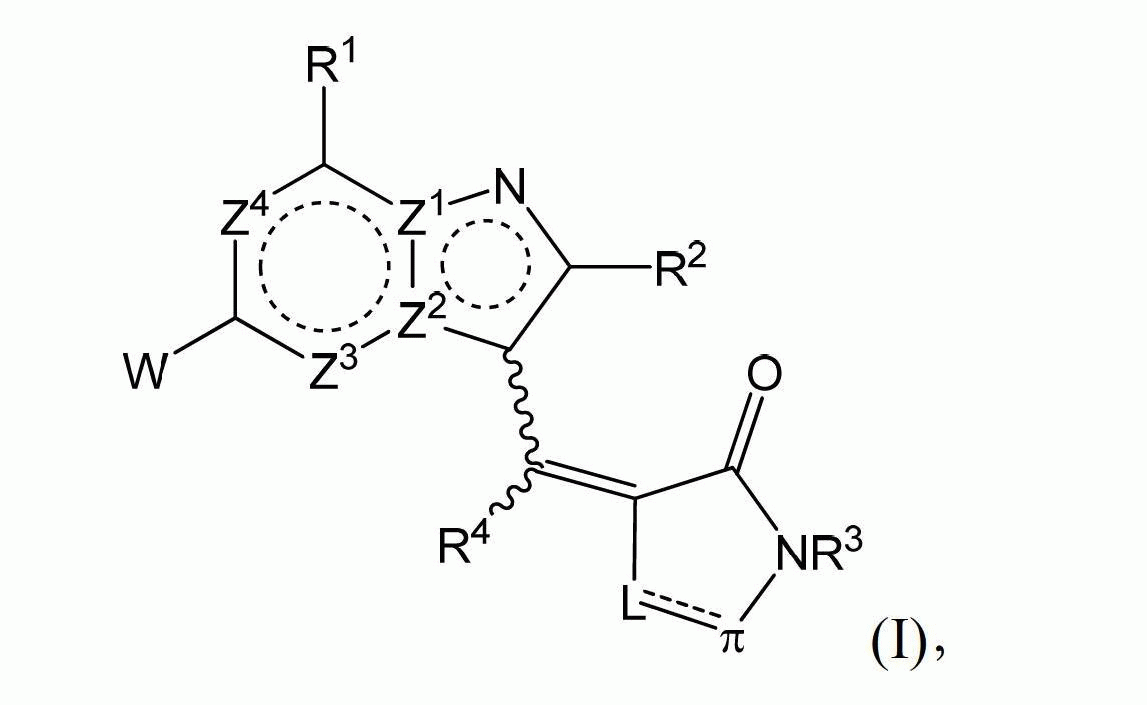
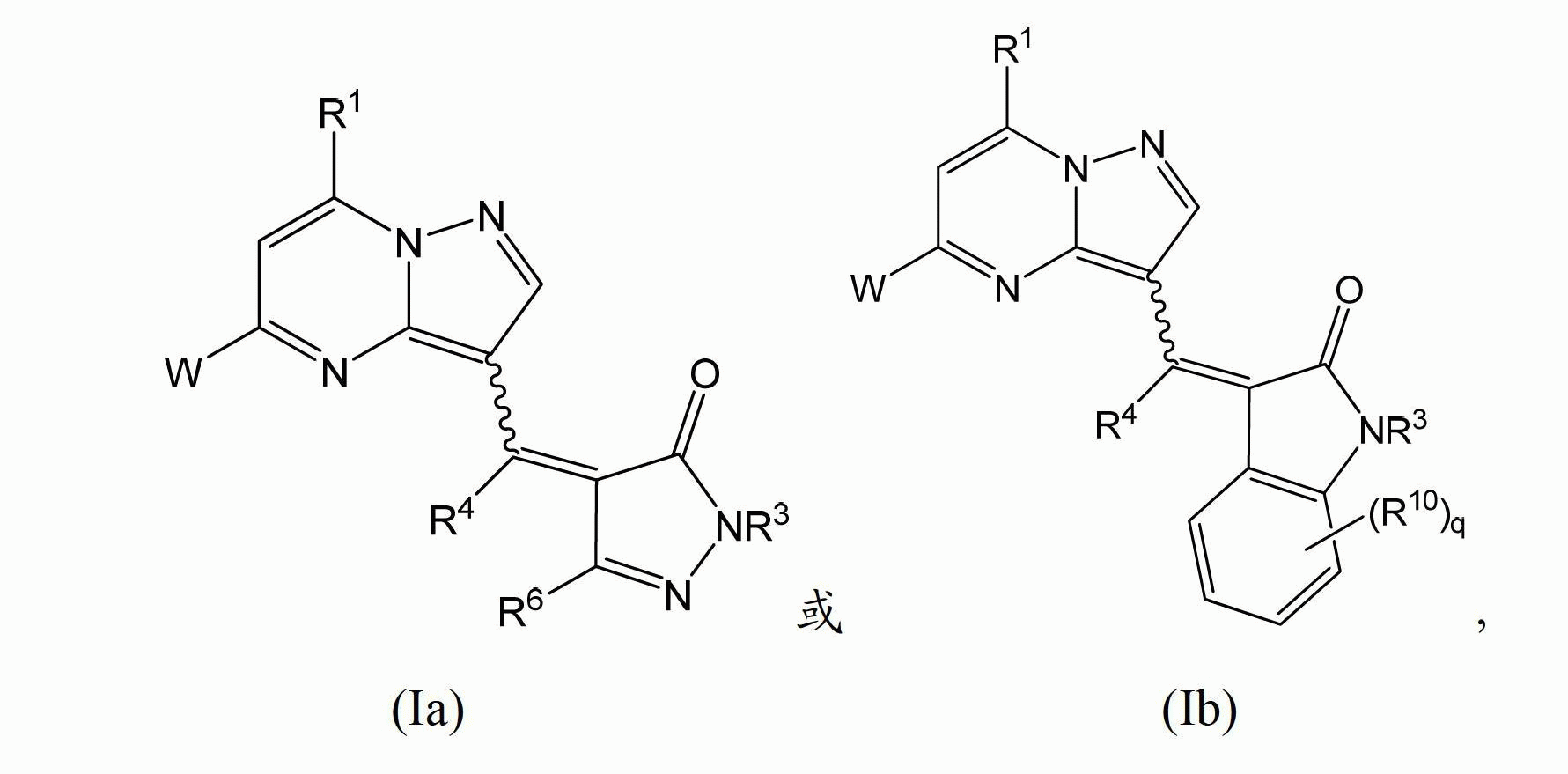
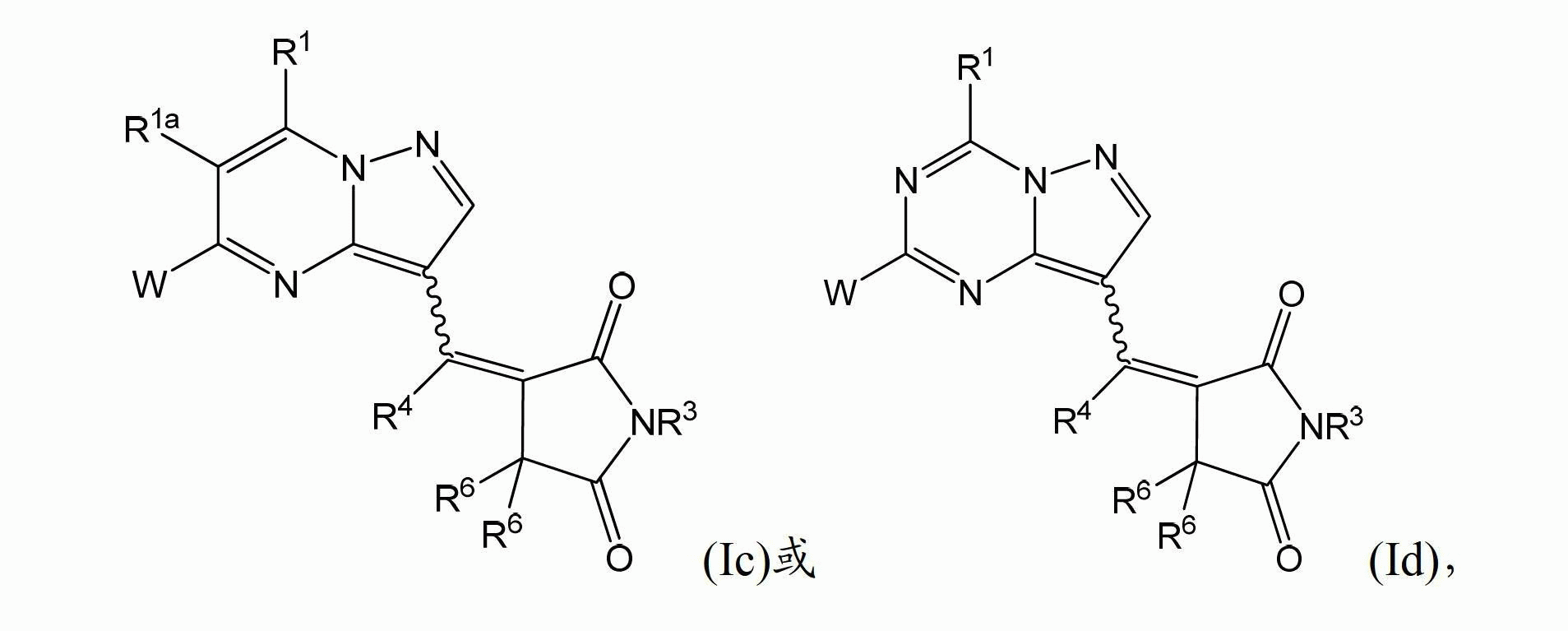
[0164] In one embodiment, the present invention provides a compound having structural formula (I):
[0165]
[0166] Or a pharmaceutically acceptable salt, solvate and / or prodrug thereof,
[0167] among them:
[0168] The double ring ring system includes Z 1 -Z 4 Is aromatic
[0169] Z 1 And Z 2 One of them is C, Z 1 And Z 2 The other one is N;
[0170] Z 3 And Z 4 Independently CR 1a Or N,
[0171] R 1 And R 1a Independently H, halo, CN, optionally substituted C1-C4 alkyl, optionally substituted C2-C4 alkenyl, optionally substituted C2-C4 alkynyl, optionally substituted C1-C4 alkoxy , Or -NR 7 R 8 ;
[0172] R 2 Is H, halo, CN, or an optionally substituted group selected from C1-C4 alkyl, C2-C4 alkenyl and C2-C4 alkynyl;
[0173] R 3 And R 4 Independently selected from H and optionally substituted C1-C10 alkyl;
[0174] π is sp 2 -Hybrid C or N;
[0175] If π is C=Y, where Y is O or S, the bond shown by the dotted line is a single bond;
[0176] Or if π is N or CR 1 , The bond shown by the ...
Embodiment 1
[0361] Synthesis of 3-((5-(3-chlorophenylamino)pyrazolo[1,5-a]pyrimidin-3-yl)methylene)-5-fluoroindoline -2-one
[0362]
[0363] Add POCl to 1.5ml DMF containing 5-chloropyrazolo[1,5-a]pyrimidine (200mg, 1.31mmol) 3 (358 μL, 3.92 mmol). The reaction was stirred overnight at room temperature. The mixture was cooled to 0°C in an ice bath and then neutralized with 6M NaOH. The formed solid was separated by filtration and air dried to obtain 165 mg of 5-chloropyrazolo[1,5-a]pyrimidine-3-carbaldehyde (70% yield) as a yellow solid. LCMS(M+1=182)
[0364]
[0365] To 1.5 ml of dioxane containing 5-chloropyrazolo[1,5-a]pyrimidine-3-carbaldehyde (120 mg, 0.66 mmol) was added 3-chloroaniline (35 μL, 3.31 mmol). The mixture was heated in the microwave at 120°C for 10 minutes. The formed solid was separated by filtration and air dried to obtain 5-(3-chlorophenylamino)pyrazolo[1,5-a]pyrimidine-3-carbaldehyde as an orange solid. LCMS(M+1=273)
[0366]
[0367] To 1mL EtOH containing 5-(3-...
Embodiment 2
[0369] Synthesis of 4-((5-(3-chlorophenylamino)pyrazolo[1,5-a]pyrimidin-3-yl)methylene)-3-methyl-1H- Pyrazole-5(4H)-one
[0370]
[0371] To 5-(3-chlorophenylamino)pyrazolo[1,5-a]pyrimidine-3-carbaldehyde (80mg, 0.294mmol) in EtOH was added 3-methyl-1H-pyrazole-5(4H )-Ketone (29 mg, 0.294 mmol) and piperidine (30 μL, 0.294 mmol). The mixture was heated at 70°C overnight. The formed solid was separated by filtration to obtain 4-((5-(3-chlorophenylamino)pyrazolo[1,5-a]pyrimidin-3-yl)methylene)-3-methyl- 1H-pyrazole-5(4H)-one. LCMS(M+1=353)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com