Method for ammonia method desulfurization of flue gas and high-purity ammonium hydrogen sulfite by-producing
A technology of ammonium bisulfite and ammonia desulfurization, applied in separation methods, chemical instruments and methods, separation of dispersed particles, etc., can solve problems such as difficulty in meeting emission requirements, ammonia escape, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
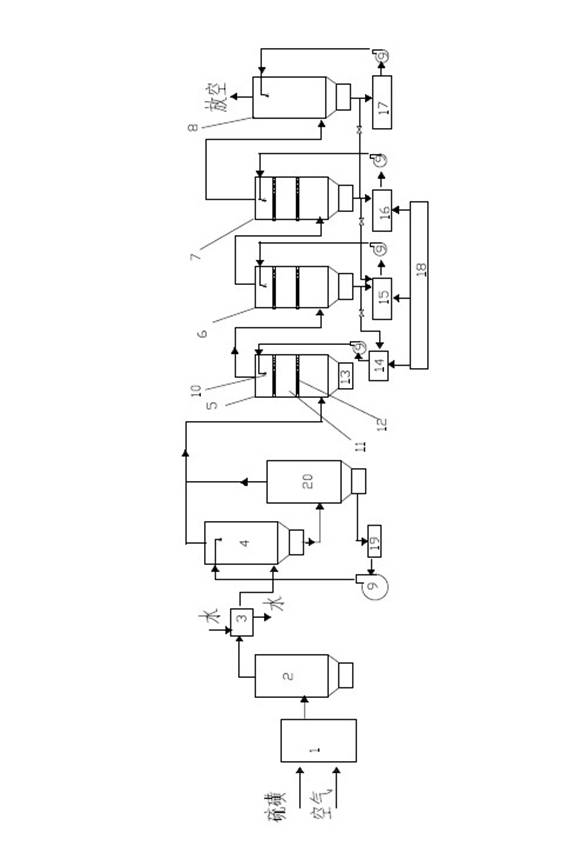
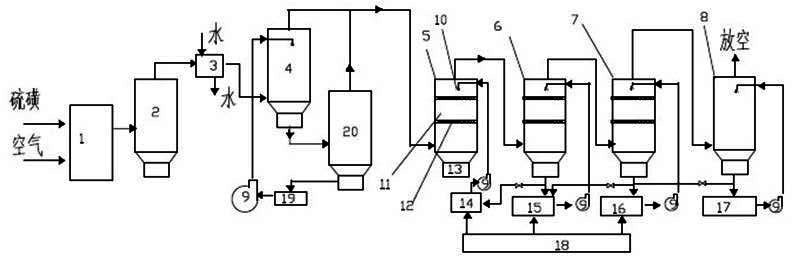
Image
Examples
Embodiment 1
[0016] Example 1: Simulated flue gas composition: 78% N 2 , 3% O 2 , 15% SO 2 , 3% CO 2 and water vapor. Flue gas flow: 1m 3 / min. The ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 5-6; the ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 6-7; the ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 6-7. The result is as follows:
[0017] Flue gas flow rate (m 3 / min) SO after purification 2 Content (ppm) Purified NH 3 Content (ppm) NH 4 HSO 3 Solution concentration 1 58 32 75%
Embodiment 2
[0018] Example 2: Simulated flue gas composition: 78% N 2 , 3% O 2 , 12% SO 2 , 7% CO 2 and water vapor. Flue gas flow: 1m 3 / min. The ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 5-6; the ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 6-7; the ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 6-7. The result is as follows:
[0019] Flue gas flow rate (m 3 / min) SO after purification 2 Content (ppm) Purified NH 3 Content (ppm) NH 4 HSO 3 Solution concentration 1 65 28 70%
Embodiment 3
[0020] Example 3: Composition of simulated flue gas: 78% N 2 , 8 of O 2 %, 8% SO 2 , 5% CO 2 and water vapor. Flue gas flow: 1m 3 / min. The ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 5-6; the ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 6-7; the ammonia water and (NH 4 ) 2 SO 3 The pH value of the solution is 5-6. The result is as follows:
[0021] Flue gas flow rate (m 3 / min) SO after purification 2 Content (ppm) Purified NH 3 Content (ppm) NH 4 HSO 3 Solution concentration 1 58 62 72%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com