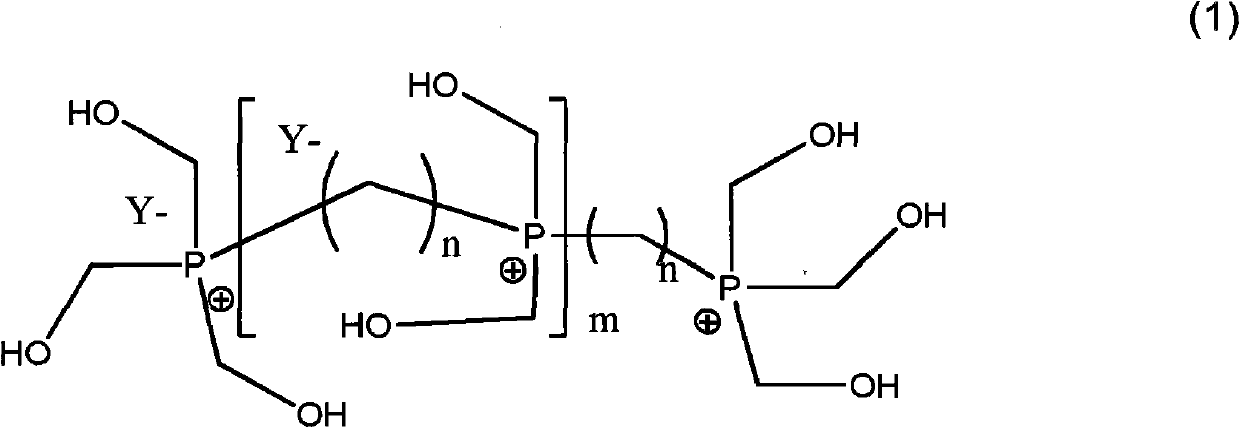
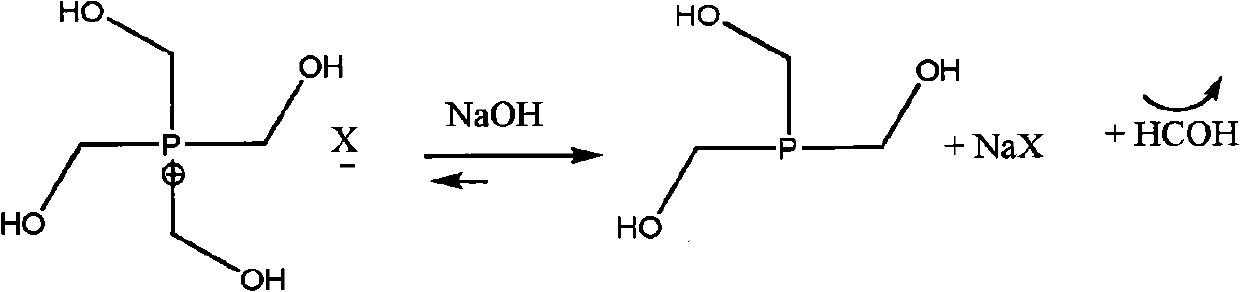
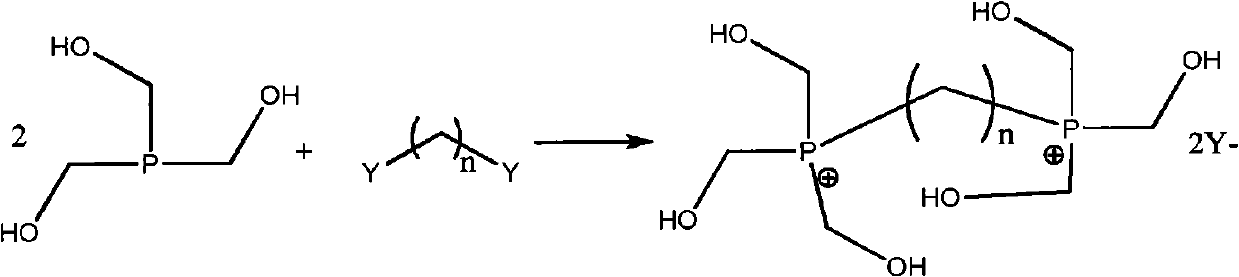
Organophosphorus derivatives and use thereof as uncoupling agents
A technology of uncoupling agent and organophosphorus, which is applied to organophosphonium derivatives. The decoupling agent is used in the field of aqueous systems, which can solve the problems of reduced biomass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Step a) the synthesis of trihydroxymethylphosphine solution
[0057] In a well-stirred reactor equipped with vacuum distillation and previously inert under nitrogen, charge:
[0058] Titrate 80% THPC (tetrakis (hydroxymethyl) phosphorus chloride) and contain the solution of 150g THPC (0.63mol);
[0059] Cool the mixture to 5-15 °C;
[0060] Pour 385g of 8% soda solution in 3 hours and control the temperature at 5-15°C;
[0061] After the end of pouring, the reaction mixture was kept at 10 °C for 12 hours;
[0062] Formaldehyde removal by distilled water / formaldehyde mixture at a temperature of 15-30°C and a pressure of <10mbar;
[0063] Add 970g of ethanol and distill under the same conditions to consume formaldehyde;
[0064] Precipitation of NaCl was observed and the reaction was analyzed after distillation:
[0065] Analysis: 93.5% THP / 3% THPO / 3.5% THPC.
Embodiment 2
[0067] Steps b) and c): Synthesis of phosphonium (formula (1), n=8, m=0 or 1)
[0068] The reactants prepared in Example 1 were introduced into a well-stirred reactor equipped with vacuum distillation and previously inertized under nitrogen. During this period, the temperature was controlled at 45-55° C., and 111.8 g of 1,8-diiodooctane was poured in within 30 minutes.
[0069] The reaction medium was biphasic and precipitation of salts (NaI / NaCl) was observed.
[0070] After 50 hours at 50° C., the reaction medium was filtered and the product obtained was a pale yellow transparent solution, from which 665 g in weight were extracted with the following NMR (nuclear magnetic resonance) analysis:
[0071] 31 P NMR analysis
[0072] 3%THPO
[0073] 3% THP
[0074] 14% THPC
[0075] 78% Organophosphonium derivatives of diphosphonium and triphosphonium according to the following molar composition:
[0076]
[0077]
Embodiment 3
[0079] Step a) the synthesis of trihydroxymethylphosphine solution
[0080] In a well-stirred reactor equipped with vacuum distillation and previously inert under nitrogen, a titrated 80% THPC (tetrakis (hydroxymethyl) phosphorus chloride) containing 100 g of THPC (0.42 mol) and water ( 50g) solution;
[0081] Cool the mixture to 5-15 °C;
[0082] Pour 228g of 8% soda NaOH solution within 3 hours and control the temperature at 5-15°C;
[0083] After pouring, keep at 10°C for 12 hours;
[0084] At a temperature of 15-30 ° C and a pressure of less than 10 mbar, the formed formaldehyde was removed by distilled water / formaldehyde mixture; 786 g of ethanol was added and distilled under the same conditions to consume formaldehyde;
[0085] Precipitation of NaCl was observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com