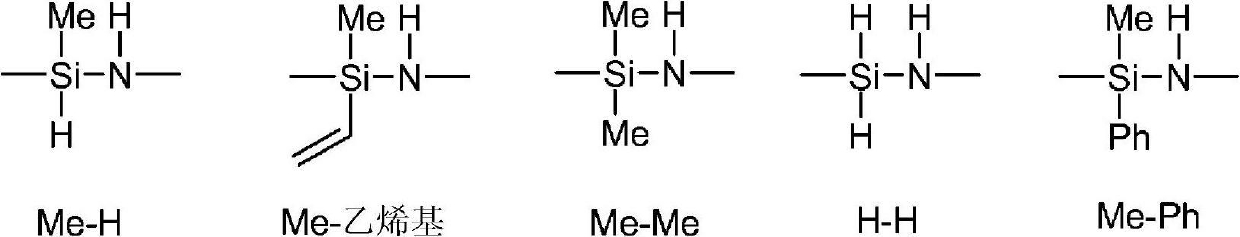Process for preparing shelf-stable curable polysilazanes, and polysilazanes prepared thereby
A technology of polysilazane and dihalosilane, which is applied in the field of preparing curable polysilazane-containing compositions, can solve problems such as high cost and large odor, and achieve convenient purification, easy storage, and high industrial compatibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
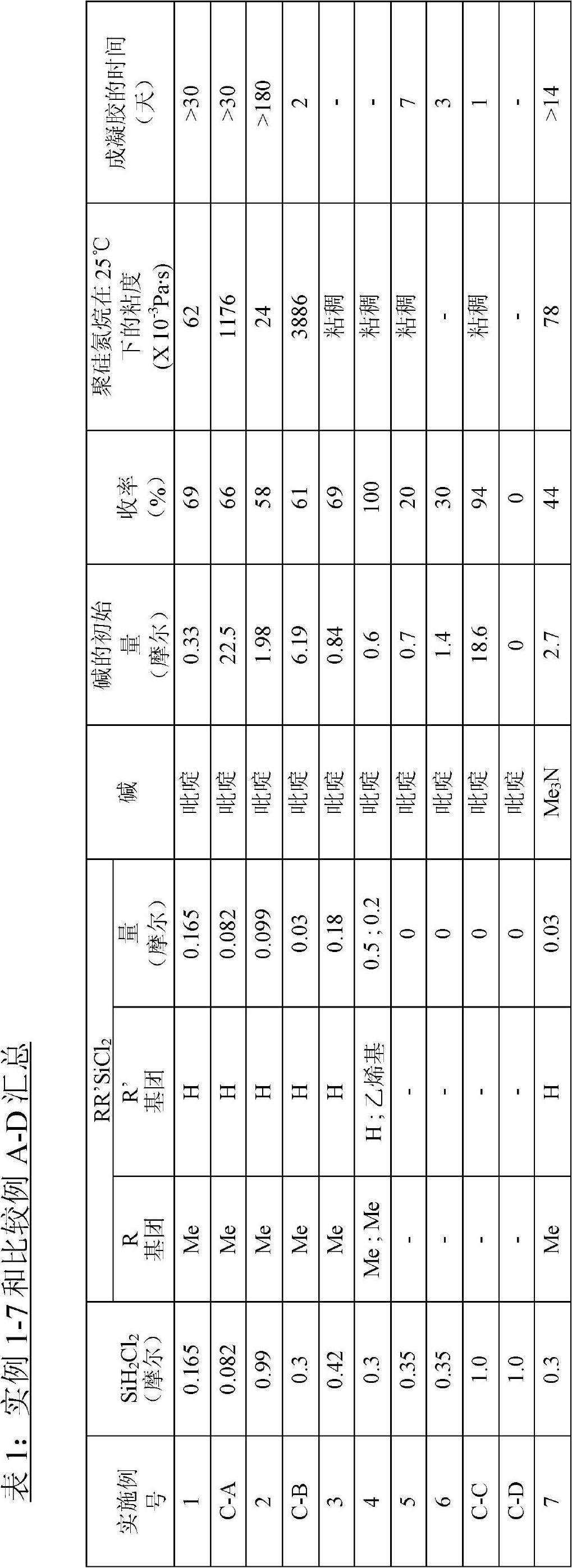
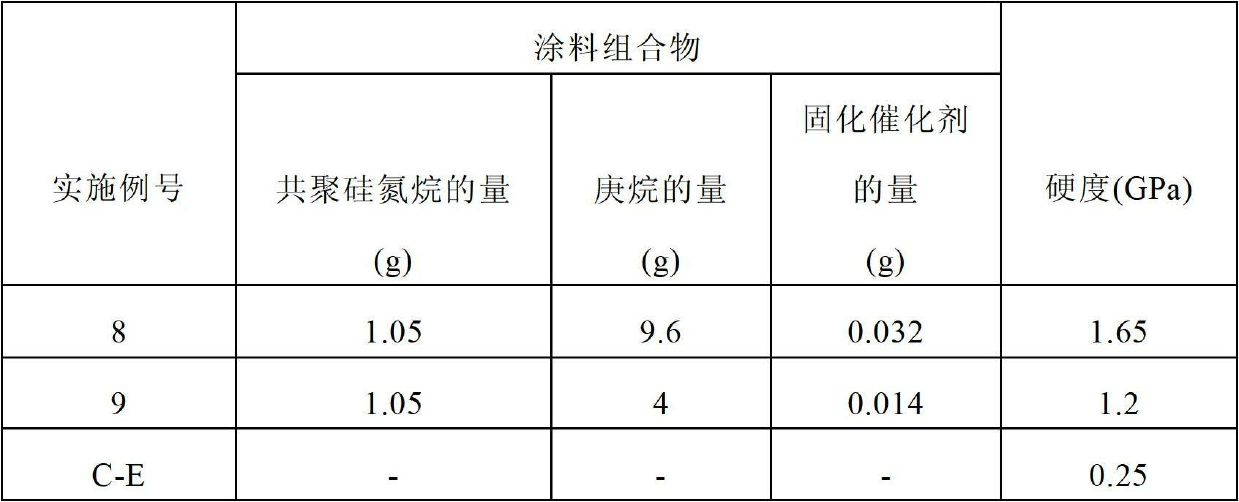
Examples
preparation example Construction
[0057] Preparation of dihalosilane-base adducts
[0058] Dihalosilane-base adducts can be prepared by combining at least one of the aforementioned dihalosilanes with at least one of the aforementioned bases. Dihalosilanes can react with bases to form adducts, and the rate of formation and their stability may depend on the acidity of the dihalosilanes and the basicity of the base. The dihalosilane and base can be selected so as to produce an adduct that is stable enough to serve as a reaction intermediate capable of reacting with ammonia or primary amines. In particular, one mole of dichlorosilane is known to react with two moles of pyridine to form a 1:2 (halide:base) adduct or with one mole of tetramethylethylenediamine to form a 1:1 adduct (as e.g. H.J.Campbell-Ferguson and E.A.V.Ebsworth described in J.Chem.Soc. (A), 1966,1508, its relevant adduct description content is incorporated herein by reference; According to Campbell-Ferguson and Ebsworth described, with Substit...
example
[0092] Objects and advantages of this invention are further illustrated by the following examples, but the particular materials and amounts thereof recited in these examples, as well as other conditions and details, should not be construed to unduly limit this invention. These examples are for illustrative purposes only and are not intended to limit the scope of the appended claims.
[0093] All parts, percentages, ratios, etc. in the examples, as well as in the remainder of the specification, are by weight unless otherwise indicated. Solvents and other reagents used were obtained from Aldrich Chemical Company, Milwaukee, WI unless otherwise indicated.
[0094] Material
[0095] Hexane was obtained from Aldrich Chemical Company, Milwaukee, WI.
[0096] Anhydrous pyridine was obtained from Aldrich Chemical Company, Milwaukee, WI.
[0097] Dichlorosilane (25% by weight in xylene) was obtained from Gelest, Inc., Morrisville, PA.
[0098] Methyldichlorosilane was obtained fr...
example 1 and comparative example A
[0106] preparation
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com