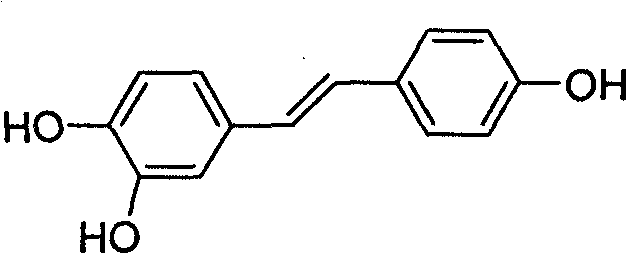
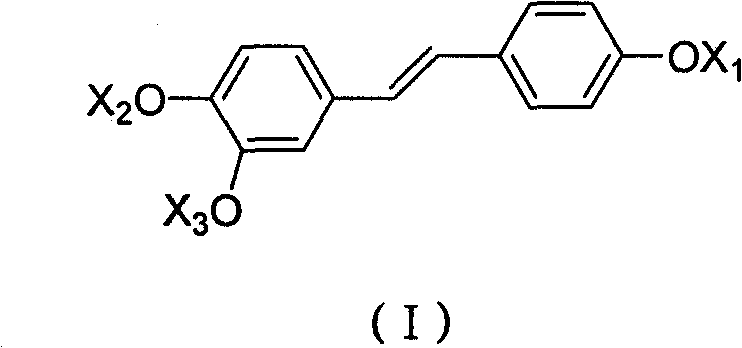
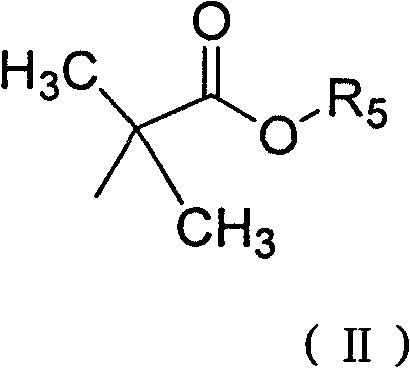
Derivative of high veratryl alcohol and medical application of derivative of high veratryl alcohol
A technology of homoveratrol and its derivatives, which is applied in the field of medicine and can solve problems such as easy oxidation of compounds, short time, unsuitable drug index, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of Ethyl Hoveratrol Trioxoisobutyrate
[0032] Take 1.12g (6.24mmol) of ethyl 2-bromoisobutyrate and put it into a three-necked flask with a stirring device, and add 20ml of DMF successively under stirring at room temperature, 0.5K 2 CO 3 , 0.05g tetrabutylammonium bromide. After stirring evenly, add 0.39g homoveratrol (1.71mmol) and 10ml DMF mixture dropwise. After the dropwise addition, raise the temperature to 55°C to continue the reaction for 5h, filter, and the filtrate is rotary evaporated under reduced pressure, and the organic solvent is recovered to obtain a yellow solid. Separation by column chromatography yielded 0.56 g of homoveratrol methyl trioxoisobutyrate with a yield of 62.3%.
[0033] 1 H-NMR (DMSO, 300MHz) 1.26 (9H, m), 1.58-1.62 (18H, m), 4.27 (6H, m), 6.26 (1H, t), 6.64 (2H, d), 6.83 (3H, m ), 6.88 (1H, m), 7.36 (2H, m), MS: 570 (M + ).
[0034] Reaction formula:
[0035]
[0036] The compound of Example 1 has been shown to be e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com