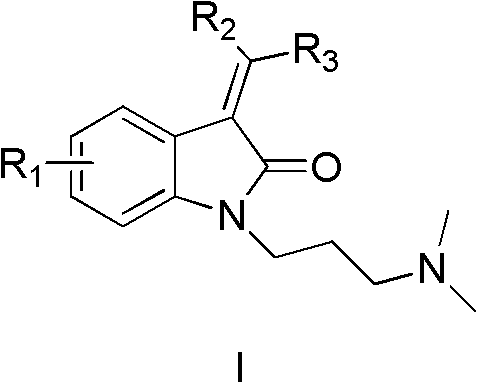
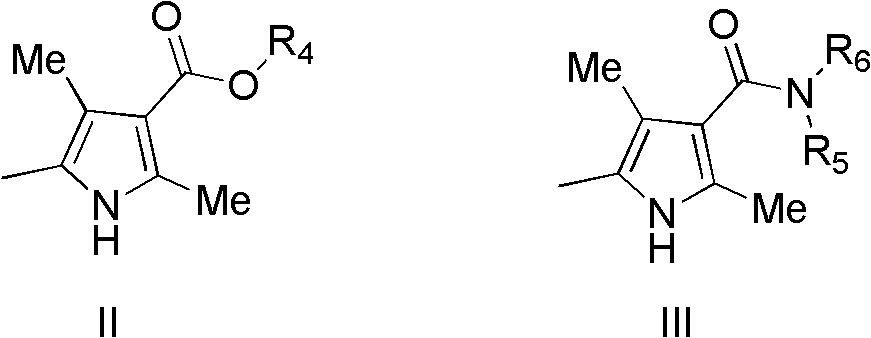
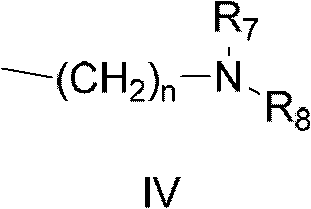Indolinone derivatives, pharmaceutical composition thereof, preparation method and application thereof
A compound, C1-C3 technology, applied in the field of medicine and chemical industry, can solve the problems of difficult large-scale production, difficult chemical synthesis, complex molecular structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0069] Preparation Example 1: Synthesis of 2-benzoyl-pyrrole
[0070] Under the protection of nitrogen, 3.00g (44.7mmol) pyrrole was dissolved in 60mL ether, 5.86g (90.1mmol) zinc powder was added under stirring at 0°C, and 5.67g (40.3mmol) benzoyl chloride was slowly added dropwise, and the reaction was completed for 1 hr. After filtration, concentration, and silica gel column chromatography, 4.00 g of a yellow-white solid was obtained, with a yield of 58.0%. 1 H-NMR (400MHz, DMSO-d6) δppm: 12.08 (brs, 1H), 7.81-7.79 (m, 2H), 7.63-7.59 (m, 1H), 7.55-7.51 (m, 2H), 7.23-7.21 ( m, 1H), 6.78-6.77 (m, 1H), 6.27-6.26 (m, 1H).
preparation example 2
[0071] Preparation Example 2: Synthesis of 2-propionyl-pyrrole
[0072] Synthesized with reference to the method of Preparation Example 1 to obtain a white solid with a yield of 61.0%. 1 H-NMR (400MHz, DMSO-d6) δppm: 11.76 (br s, 1H), 7.06-7.04 (m, 1H), 6.97-6.95 (m, 1H), 6.16-6.18 (m, 1H), 2.72-2.89 (q, 2H, J=7Hz), 1.08-1.04 (t, 3H, J=7Hz).
preparation example 3
[0073] Preparation 3: 3,5-Dimethyl-1H-pyrrole-2,4-dicarboxylic acid-2-tert-butyl-4-ethyl ester synthesis
[0074] Under cooling in an ice-water bath, dissolve 8.35g (52.8mmol) of tert-butyl acetoacetate in 15mL of glacial acetic acid, and add dropwise an aqueous solution of sodium nitrite (4.50g of sodium nitrite in 10mL of water) while stirring. over 10°C. After dropping, stir at room temperature for 3 hrs to obtain a light yellow transparent solution, which is set aside for use.
[0075] Dissolve 5.87g (45.1mmol) of ethyl acetoacetate in 20mL of glacial acetic acid and heat to 65°C. Slowly add the light yellow transparent solution prepared above dropwise, and add 10.37g of zinc powder in 5 times. The process is exothermic, and the temperature control does not exceed 80°C. After the addition was complete, the temperature was raised to 75° C. for 1 hr. After cooling to room temperature, a white solid precipitated and was filtered. Petroleum ether was recrystallized to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com