Medicine composition prepared from alanyl glutamine and compound amino acid by utilizing spray drying method
A technology of alanyl glutamine and compound amino acids, applied in the field of medicine, can solve the problems of unfavorable wide application of alanyl glutamine and amino acids, great difference in drug quality, influence on drug quality, etc., and achieves improvement of clinical application quality. and bioavailability, ease of procurement and dispensing, and efficacy in reducing liver damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of Combination Packaged Drugs
[0040] Composition: Alanyl glutamine sterile powder for injection 10g, water for injection 50ml and compound amino acid injection 250ml
[0041] making process:
[0042] 1. Preparation of Alanyl Glutamine for Injection
[0043] (1) Dissolve 1000g of alanylglutamine and 5000ml of water for injection in the batching room of the 10,000-class clean area to make a solution of about 20%, add 15g of medicinal activated carbon, stir and absorb for 30 minutes, and decarbonize by coarse filtration. After fine filtration through a 0.22 μm microporous membrane, the filtrate was spray-dried to obtain alanyl glutamine sterile powder.
[0044] (2) Class 100 clean area, under the condition of class 100, the relative humidity is controlled to be lower than 45%, and the automatic packaging machine is used for aseptic packaging, 10g / bottle, stoppered, and capped.
[0045] 2. Preparation of water for injection
[0046] Fill with fr...
Embodiment 2
[0053] Example 2 Preparation of Combination Packaged Drugs
[0054] Combination: 20g of alanyl glutamine powder for injection, 100ml of water for injection and 500ml of compound amino acid injection
[0055] making process:
[0056] 1. Preparation of Alanyl Glutamine for Injection
[0057] (1) Dissolve 2000g of alanylglutamine and 10000ml of water for injection in the batching room of the 10,000-class clean area to make a solution of about 20%, add 30g of medicinal activated carbon, stir and absorb for 30 minutes, and decarbonize by coarse filtration. After fine filtration through a 0.22 μm microporous membrane, the filtrate was spray-dried to obtain alanyl glutamine sterile powder.
[0058] (2) Class 100 clean area, under the condition of class 100, the relative humidity is controlled to be lower than 45%, and the automatic filling machine is used for aseptic subpackaging, 20g / bottle, stoppered and capped.
[0059] 2. Preparation of water for injection
[0060] Fill wit...
experiment example 3
[0067] Experimental example 3 Stability test
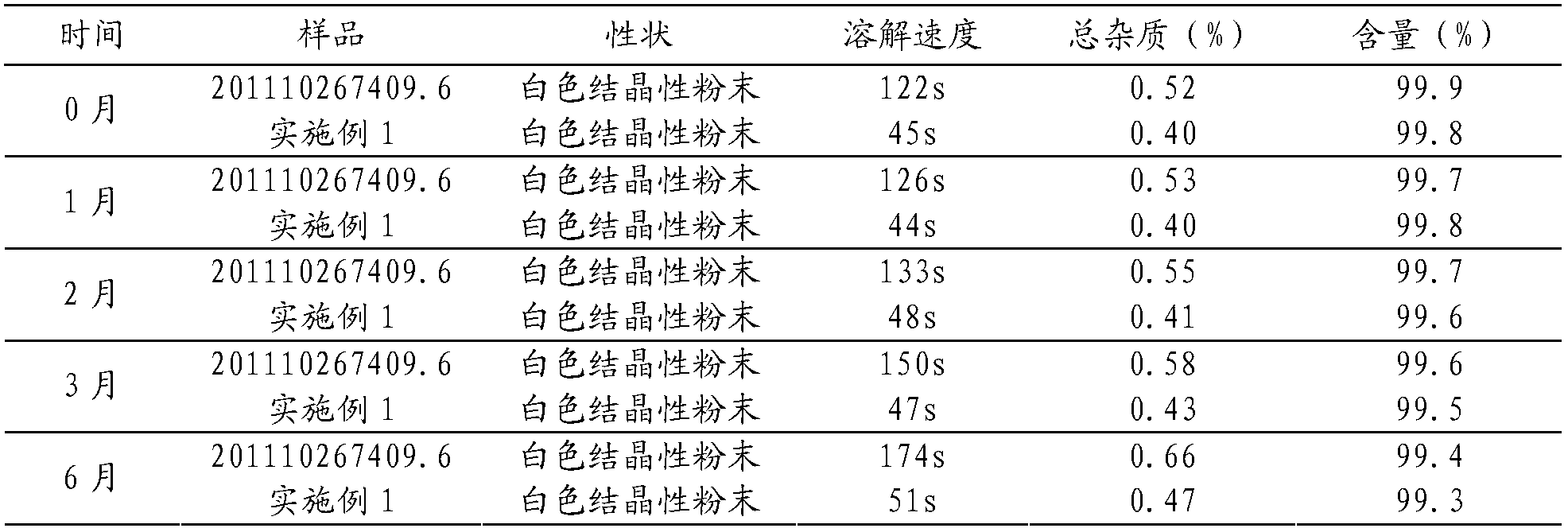
[0068] A batch of samples were prepared according to Example 1 of 201110267409.6, and compared with the samples prepared in Example 1 of the present invention, an accelerated test was carried out at a high temperature of 40°C and a relative humidity of 75%±5% for 6 months. The results are shown in Table 1.
[0069] Table 1 Stability and dissolution rate determination results
[0070]
[0071] As can be seen from Table 1, the dissolution rate of the sample prepared in Example 1 of 201110267409.6 is significantly slow, and the relevant substances increase during the acceleration of 6 months; while the dissolution rate of the sample prepared in Example 1 of the present invention is very fast, and the relevant substances change after 6 months of acceleration smaller. It fully demonstrates the superiority of the present invention in improving stability and dissolution rate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com