Asiaticoside external use sterile powder and its preparation method
A technology of asiaticoside and asiaticoside total glucoside, which is applied in the field of external use solid sterile preparation of asiaticoside and its preparation field, can solve the problems such as inability to fully exert the medicinal effect, inability to ensure the cleaning of the wound surface, use restriction and the like, and achieves the The effect of reducing drug decomposition products, facilitating storage at room temperature, and improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] The composition of the preparation: 62.5g of Centella asiatica total glycosides (reduced pure), 20.0g of sodium lauryl sulfate, and water for injection to 1000ml.
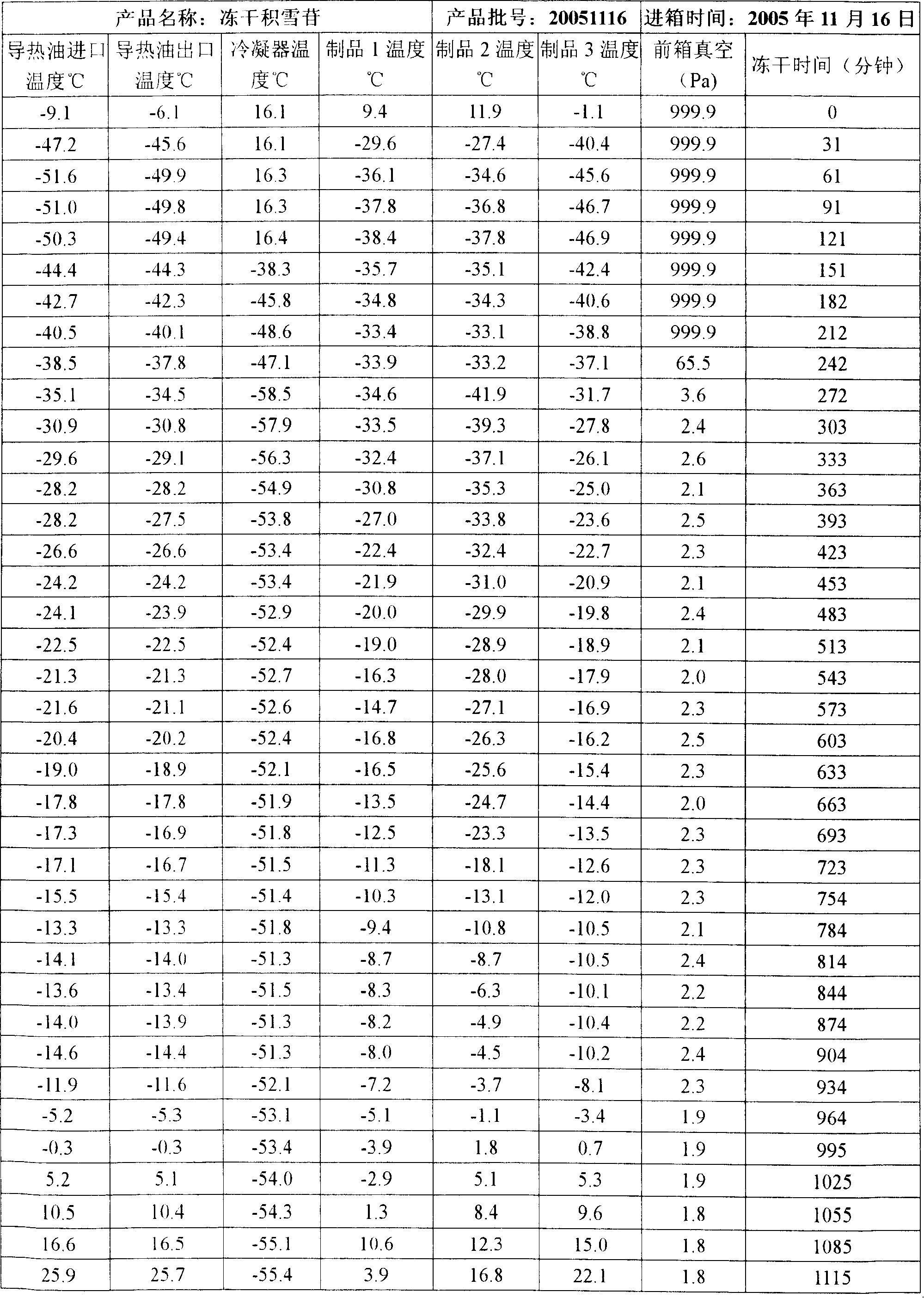
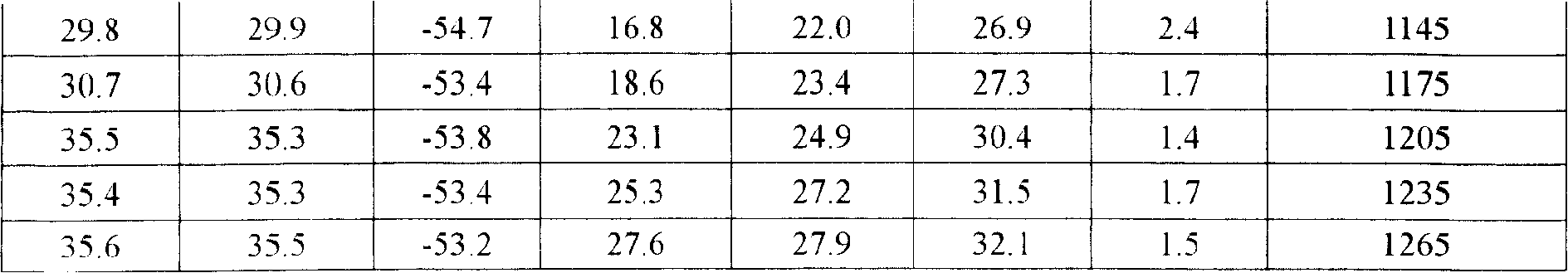
[0012] Preparation method: Weigh the raw material of Centella asiatica total glycosides, add 80% water for injection, heat and stir to dissolve, coarse filter with sand filter, then add sodium lauryl sulfate, stir to dissolve, adjust pH with 1mol / L HCl Value to 6.0, add 0.2% activated carbon for needles, keep at 60℃ for 30 minutes, coarse filter with sand filter rod, make up the solution, filter with 0.45μm membrane, filter with 0.22μm microporous sand core, fill and freeze-dry according to the setting The curve is freeze-dried (see Freeze-Drying Table 1), stoppering, capping, printing and packaging. After the product is fully inspected, it will be put into storage to obtain the freeze-dried asiaticoside for external use. The specification is 0.25g / bottle.
[0013] In the preparation of the present invention, th...
Embodiment 2
[0017] Example 2 Selection of Cosolvent
[0018] The total glycosides of asiatica contains many poorly water-soluble components. When heated, these components can be dissolved in water, and then precipitated out after cooling. Adjusting the pH value to alkaline (pH>9) can also increase the solubility of these ingredients, but considering the irritation of the preparation to the skin, it is difficult to adjust the pH value in the process. Therefore, it is necessary to select an appropriate co-solvent to increase the solubility of these ingredients, especially the effective ingredients.
[0019] A variety of cosolvents have been studied. The research plan and results are shown in Table 2:
[0020] Cosolvent
[0021] It can be seen that sodium lauryl sulfate and urotropine have a certain solubilizing effect on Centella asiatica total glycosides, while other reagents have weak or no solubilizing effect. In further comparison, the solubilizing effect of sodium lauryl sulfate ...
Embodiment 3
[0022] Example 3 Preparation method research
[0023] The composition of the total glucosides of Asiatic asiatica is complex, and the present invention is first dissolved in hot water for injection and then coarsely filtered, which can reduce the difficulty of filtration in subsequent processes. The subsequent steps basically refer to the process of lyophilized powder preparation of traditional Chinese medicine.
[0024] Three batches of samples were prepared according to the selected formulation composition and method, with good reproducibility, consistency between batches, and meeting production requirements. The results are shown in Table 3.
[0025] Main equipment: Lyo-7.5 freeze dryer (Shanghai Dongfulong Technology Co., Ltd.)
[0026] DGS8 / 2 filling and stoppering machine
[0027] ZG-300 Capping Machine
[0028] table 3
[0029] Lot number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com