A kind of usnic acid nano-suspension and its preparation method and application
A nano-suspension, usnic acid technology, applied in the directions of liquid delivery, emulsion delivery, antibacterial drugs, etc., can solve the problems of poor medicine properties, low solubility of usnic acid, etc., and achieves less excipients, good application value, Simple and feasible preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation one of usnic acid nanocrystal suspension
[0046] The suspension of usnic acid nanocrystals was prepared by the medium dispersion method (grinding method).
[0047] prescription:
[0048]
[0049] Formulation process:
[0050] In a 100ml flask, accurately weigh about 1.6g of HPC-SL and place it in 45ml of distilled water to swell completely to obtain solution A; accurately weigh about 0.03g of SDS and dissolve it in 20ml of distilled water to obtain solution B; Mix well to obtain solution C.
[0051] Accurately weigh about 8 g of usnic acid that has passed through an 80-mesh sieve, slowly add it to solution C and continuously stir to make it evenly dispersed, and prepare a usnic acid coarse suspension for later use.
[0052] start up Mill LAB grade wet mill (Mill Research Lab, Switzerland WAB Machinery Co., Ltd.), pour the above-prepared usnic acid coarse suspension into the hopper, rinse with 25 ml of distilled water, and add it to the h...
Embodiment 2
[0056] Example 2: Preparation two of usnic acid nanocrystal suspension
[0057] The usnic acid nanocrystal suspension was prepared by nanosedimentation method (solvent diffusion method).
[0058] prescription:
[0059] Aqueous phase - 1.5% polyvinyl alcohol (W / V) and 0.5% sodium lauryl sulfate (W / V) aqueous solution, 20ml;
[0060] Oil phase - 0.8% usnic acid and 3% polylactic acid in ethyl acetate, 5ml.
[0061] Preparation Process:
[0062] Take the prescribed amount of water phase, continue stirring (1000rmp / min), slowly drop the oil phase solution into the water phase, and after ultrasonic probe treatment for 90 seconds (ice bath), homogenize three times by high pressure homogenizer APV2000. The obtained emulsion was stirred at room temperature (600 rmp / min) for 8 hours, and the ethyl acetate was evaporated to obtain it.
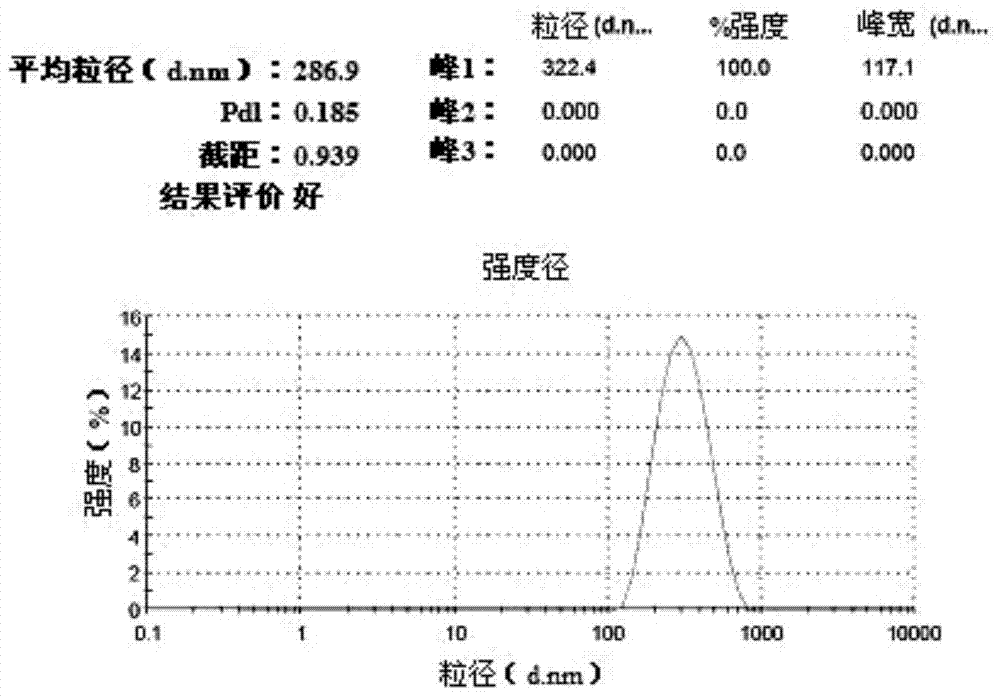
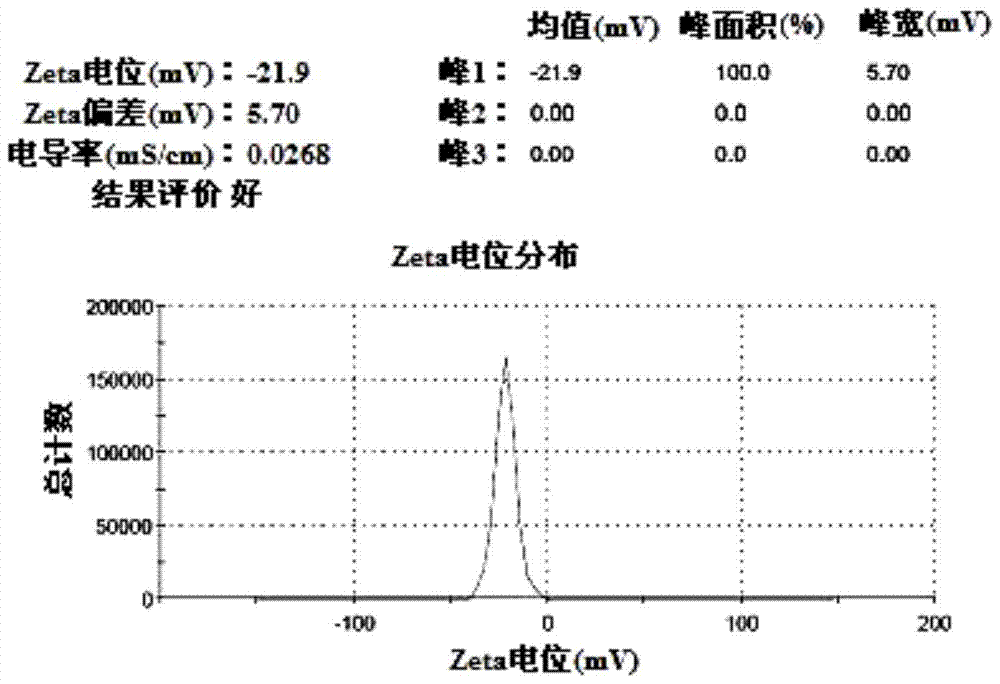
[0063] According to the particle size and potential measurement method of Example 1, the average particle size of the obtained suspension was measur...
Embodiment 3
[0064] Example 3: Freeze-drying test of usnic acid nanocrystal suspension
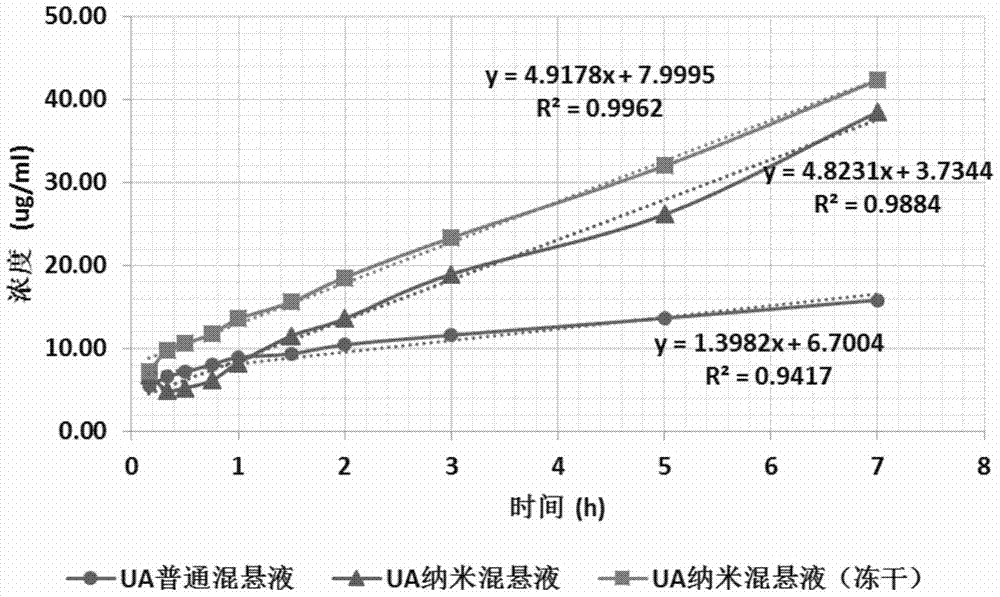
[0065] Long-term storage of the suspension is not only detrimental to the stability of the drug, but also may cause the problem of particle focusing and sedimentation, so we lyophilized the prepared usnic acid nanosuspension. Glucose or mannitol were selected as the lyoprotectant, and the freeze-drying test was carried out at the proportions of 1%, 2% and 4% (g / 100mL) respectively.
[0066] The CHRIST laboratory small freeze-drying machine was used to carry out the test, and 10 ml of the usnic acid nanosuspension prepared in Example 1 was taken, and the proportions listed in the following table 1 were respectively added with an appropriate freeze-drying protective agent, and After adding 10ml of distilled water, shake it to fully dissolve, and divide it into 5ml vials (with a thickness of 10-15mm). After pre-freezing at -80 °C overnight, take it out and place it in a freeze dryer, and slowly heat up fr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com