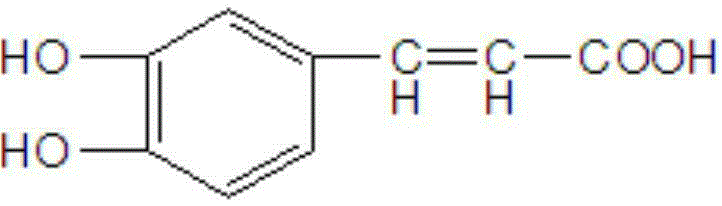
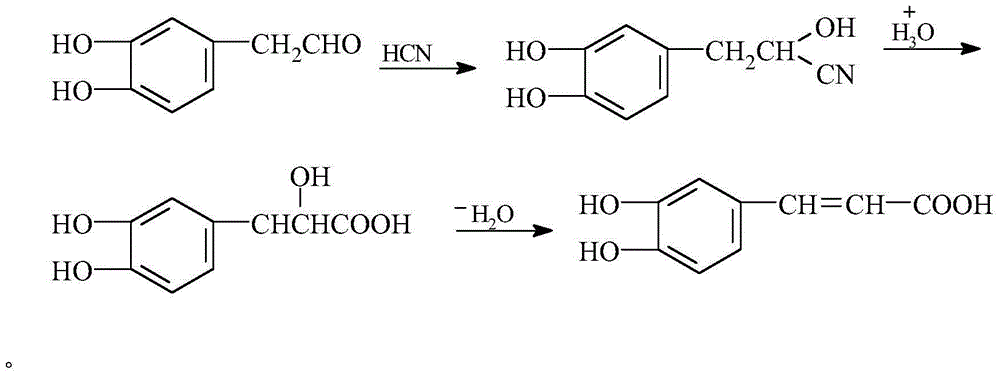
Preparation method of caffeic acid
A technology of caffeic acid and hydrocyanic acid, which is applied in the direction of carboxylate preparation, organic compound preparation, chemical instruments and methods, etc., can solve the problems of low yield, long production cycle, harsh reaction conditions, etc., and reduce production cost , Simplify the production process, reduce the effect of production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] The first step is to dissolve 500Kg of 3,4-dihydroxyphenylacetaldehyde into 0.5kg of DBU(1,8-diazabicyclo[ 5.4.0] Undec-7-ene) in PEG-200 (polyethylene glycol 200); under the conditions of 35°C and 8Mpa, 1000kg of hydrogen cyanide gas is introduced within 3 hours; hydrogen cyanide gas is introduced Start to enter ScCO after 1h 2 (Supercritical carbon dioxide) Extract the addition product to obtain the addition product;
[0024] In the second step, under the conditions of microwave radiation with a power of 500w and a frequency of 2500Hz, the addition product is hydrolyzed at 90℃ and pH 3 for 2 hours, then vacuum distillation is used to precipitate the hydrolysate, and then rinsed with acidic water for 2-3 After drying under reduced pressure, the hydrolyzate is obtained;
[0025] In the third step, under the conditions of microwave radiation with a power of 1500W and a frequency of 2000Hz, the hydrolyzed product circulates through the solid acid complex γ-Al at 120°C and -0....
Embodiment 2
[0027] The first step is to dissolve 500Kg of 3,4-dihydroxyphenylacetaldehyde into 5kg of DBNPA (2,2-dibromo-3-cyanoacetaldehyde) under microwave radiation with a power of 1200w and a frequency of 1500Hz. Propionamide) in PEG-200 (polyethylene glycol 200); under the conditions of 20°C and 10Mpa, 1200kg of hydrocyanic acid gas is introduced within 5 hours; ScCO is introduced after 1 hour of hydrocyanic acid gas 2 (Supercritical carbon dioxide) Extract the addition product to obtain the addition product;
[0028] In the second step, under the condition of microwave radiation with power of 700w and frequency of 3500Hz, the addition product is hydrolyzed at 50℃, pH value 2 for 3h, then vacuum distillation is carried out to precipitate the hydrolysate, and then rinsed with acidic water for 2-3 After drying under reduced pressure, the hydrolyzate is obtained;
[0029] In the third step, under the conditions of microwave radiation with a power of 800w and a frequency of 2500Hz, the hydrol...
Embodiment 3
[0031] The first step is to dissolve 500Kg of 3,4-dihydroxyphenylacetaldehyde in PEG-200 (polyethylene glycol) premixed with 1kg of tetrabutylammonium hydroxide under microwave radiation with a power of 1800w and a frequency of 3000Hz. 200); Under the condition of 25℃ and 16Mpa, 1500kg of hydrocyanic acid gas is introduced within 8 hours; ScCO is introduced after 1 hour of hydrocyanic acid gas 2 (Supercritical carbon dioxide) Extract the addition product to obtain the addition product;
[0032] In the second step, under the conditions of microwave radiation with a power of 900w and a frequency of 3000Hz, hydrolyze the addition product at 60℃ and pH 3 for 1 hour, and then distill under reduced pressure to precipitate the hydrolysate, and then rinse with acidic water for 2-3 After drying under reduced pressure, the hydrolyzate is obtained;
[0033] In the third step, under the conditions of microwave radiation with a power of 1200w and a frequency of 3000Hz, the hydrolyzed product is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com