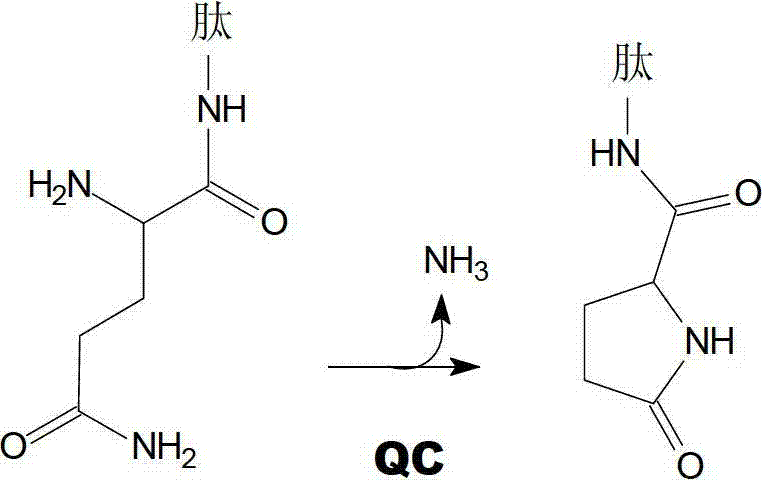
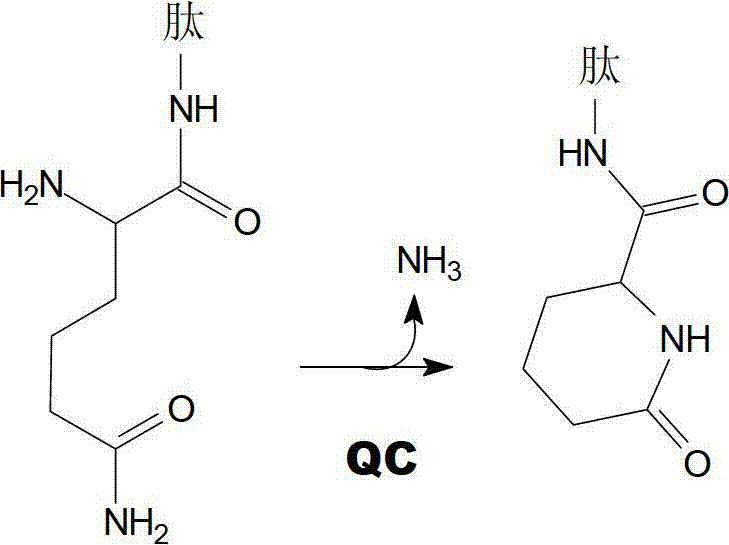
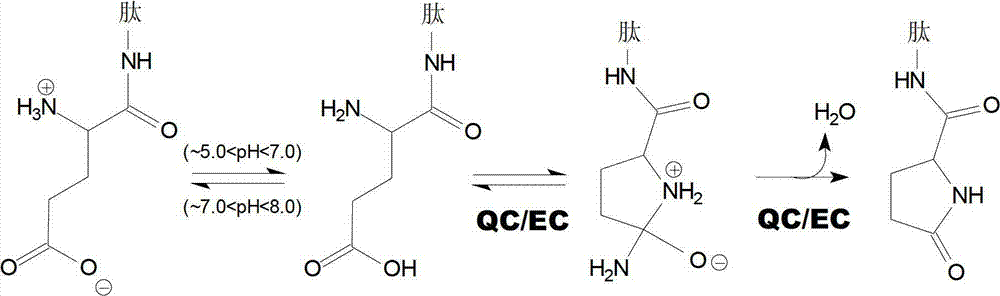
Heterocyclic inhibitors of glutaminyl cyclase (QC, EC 2.3.2.5)
A technology of heterocyclic groups and compounds, applied in the field of new heterocyclic derivatives, can solve problems such as undisclosed sequence homology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0369] Example 1: 1-(1H-benzo[d]imidazol-6-yl)-5-cyclohexyl-3-methoxy-4-methyl-1H-pyrrol-2(5H)-one
[0370] From KOH (15eq in water), N-methyl-N-nitroso-p-toluenesulfonamide (8eq), ethylene glycol / Et 2 O(1 / 2v / v, 30ml), 1-(1H-benzo[d]imidazol-6-yl)-5-cyclohexyl-3-hydroxy-4-methyl-1H-pyrrole-2(5H) - Ketone (1.00g, 3.22mmol, 1eq) and MeOH (10ml) started to synthesize the compound; Yield: 0.250g (25%); MS m / z: 326.1[M+H] + ; 1 H-NMR: (400MHz, DMSO-D 6 )δ:1.05(d,3H),1.40(d,2H),1.65-1.60(m,4H),2.05(s,3H),4.03(s,3H),4.40(s,1H),7.05(s ,1H),7.62(s,1H),7.63(s,1H),7.82(s,1H);HPLC (method [A]):rt 11.25min(98.78%)
Embodiment 2
[0371] Example 2: 1-(1H-benzo[d]imidazol-6-yl)-5-isopropyl-3-methoxy-4-methyl-1H-pyrrol-2(5H)-one
[0372] From KOH (15eq in water), N-methyl-N-nitroso-p-toluenesulfonamide (8eq), ethylene glycol / Et 2 O(1 / 2v / v, 30ml), 1-(1H-benzo[d]imidazol-6-yl)-5-isopropyl-3-hydroxy-4-methyl-1H-pyrrole-2(5H )-ketone (0.500g, 1.83mmol, 1eq) and MeOH (10ml) started to synthesize the compound; Yield: 0.040g (7.6%); MS m / z: 286.1[M+H] + ;1H-NMR: (400MHz, DMSO-D 6 )δ:0.54(d,3H),0.94-0.92(q,3H),1.95(t,3H),3.86(s,1H),4.73(s,1H),7.26-7.15(m,1H),7.65 -7.51(m,2H),8.22(d,1H),12.46(d,1H);HPLC(method [A]):rt 8.32min(96.64%)
Embodiment 3
[0373] Example 3: 1-(1H-benzo[d]imidazol-5-yl)-5-(2,6-difluorophenyl)-3-methoxy-4-methyl-1H-pyrrole-2 (5H)-Kone
[0374] From KOH (15eq in water), N-methyl-N-nitroso-p-toluenesulfonamide (5eq), ethylene glycol / Et 2 O(3 / 1v / v), 1-(1H-benzo[d]imidazol-6-yl)-5-(2,6-difluorophenyl)-3-hydroxy-4-methyl-1H- Pyrrol-2(5H)-one (0.189g, 0.5mmol, 1eq) and MeOH / H 2 O(90 / 10v / v) started to synthesize the compound; Yield: 0.058g(32.6%); MS m / z:356.3[M+H] + ; 1 H-NMR: (400MHz, DMSO-D 6 )δ:1.65-1.81(s,3H),3.83-3.93(s,3H),6.14(s,1H),6.83-7.78(m,6H),8.15(s,1H),12.07-12.07(bs, 1H); HPLC (method [A]): rt 11.24min (99%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com