Anti-HER3 antibodies and uses thereof
An antibody and expression vector technology, applied in the field of antibodies that bind to human HER3, which can solve problems such as incomplete characterization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] immunity
[0141] NMRI mice were immunized with hHER3-ECD (own use) and boosted with huHER3-ECD. The immune response was monitored by testing serum samples against HER1 / 2 / 3-ECD-ELISA. Splenocytes from mice with sufficient anti-HER3 immunoglobulin titers were frozen for later immortalization by fusion with the mouse myeloma cell line P3X63 Ag8.653. One fusion was done, and hybridoma supernatants were screened by HER1 / 2 / -ECD-ELISA that did not show cross-reactivity but combined with HER3-ECD, and anti-HER3 selective hybridomas were selected. Clonal related hybridomas were sorted by single-cell FACS. Single cell clones from different hybridomas were cultured in vitro to produce antibodies in tissue culture for characterization. Antibodies were selected by assaying their ability to inhibit HER3 phosphorylation, AKT phosphorylation, and tumor cell proliferation of MDA-MB-175 cells (see Examples below). From the obtained antibodies, one antibody was further humanized to g...
Embodiment 2
[0146] binding assay
[0147] a) Antigen-specific ELISA for binding to human HER3 ECD
[0148] Soluble human HER3 ectodomain fused to streptavidin binding protein (SBP) was captured on streptavidin plates. In order to define the optimal binding of the antibody to SPB-CDCP1, pure and step-wise diluted HEK293 supernatant (in BSA / IMDM buffer: 100 mg / ml BSA fraction V, Roche 10735078001, cultured in Iscove's modified Dulbeccos 384-well polystyrene plates (NUNC, streptavidin-coated) supplied by MicroCoat, Bernried, Germany (ID-No. 1734776-001). Using a mouse calibration curve for the chimeric 205 antibody, the optimal dilution factor of HEK293 supernatant relative to the streptavidin binding capacity of the microtiter plate was identified. For standard coating, HEK293 supernatants containing SBP-HER3 were diluted (between 1:15 and 1:40) and incubated overnight at 2-80C (25 μl per well). Vigorous washing of the microtiter plate is necessary to remove remaining unbound SBP-HER3. ...
Embodiment 3
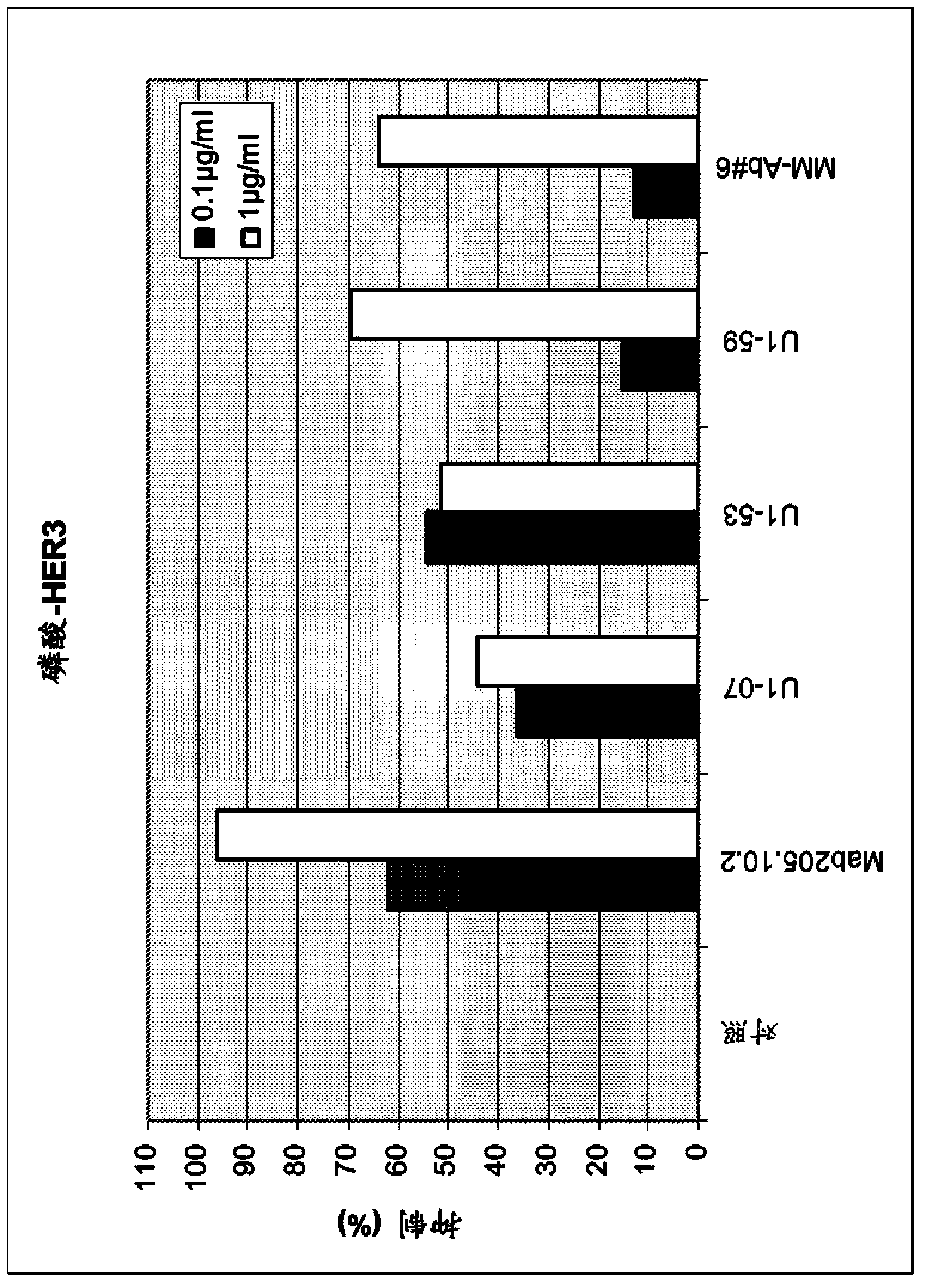
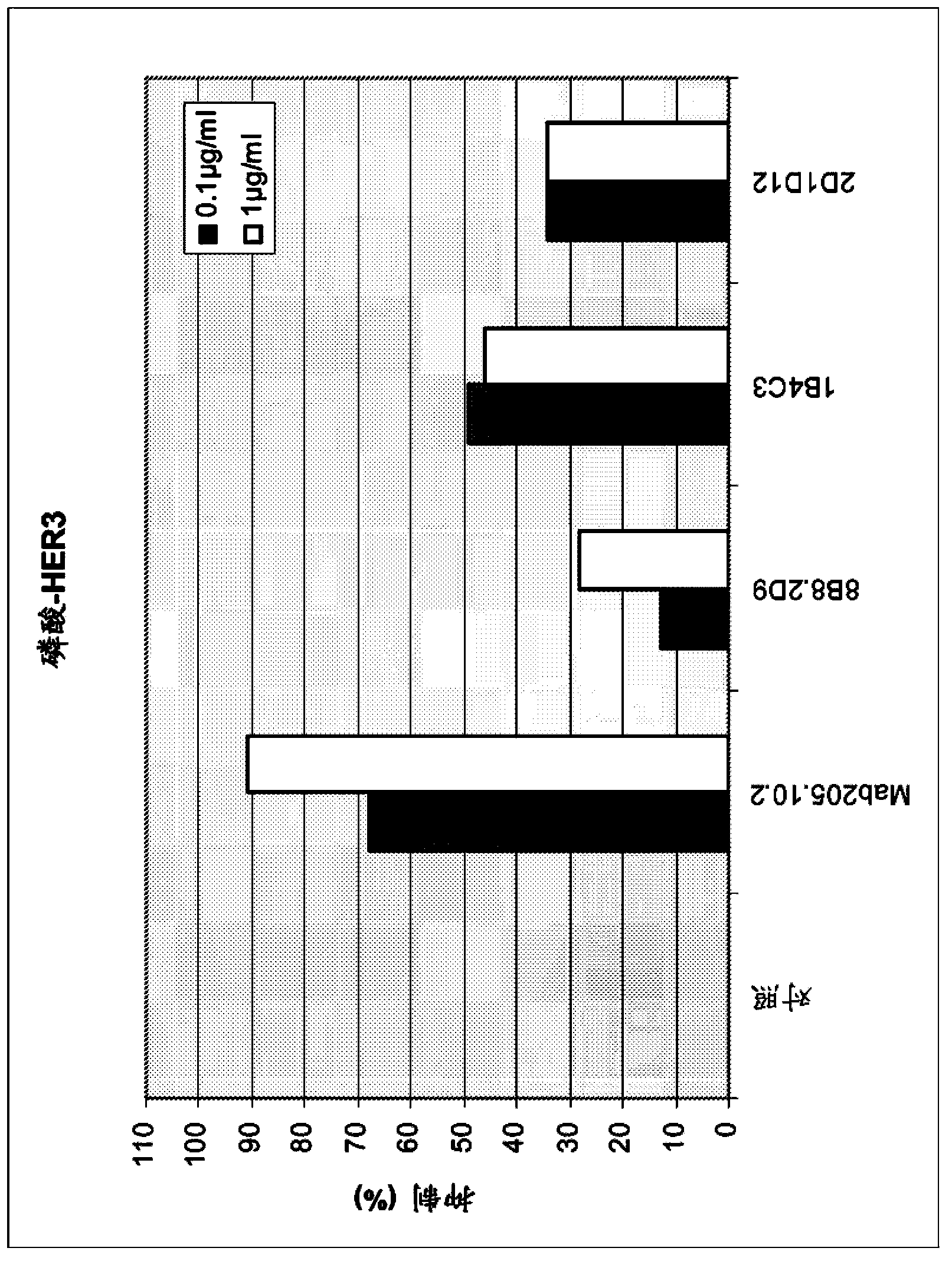
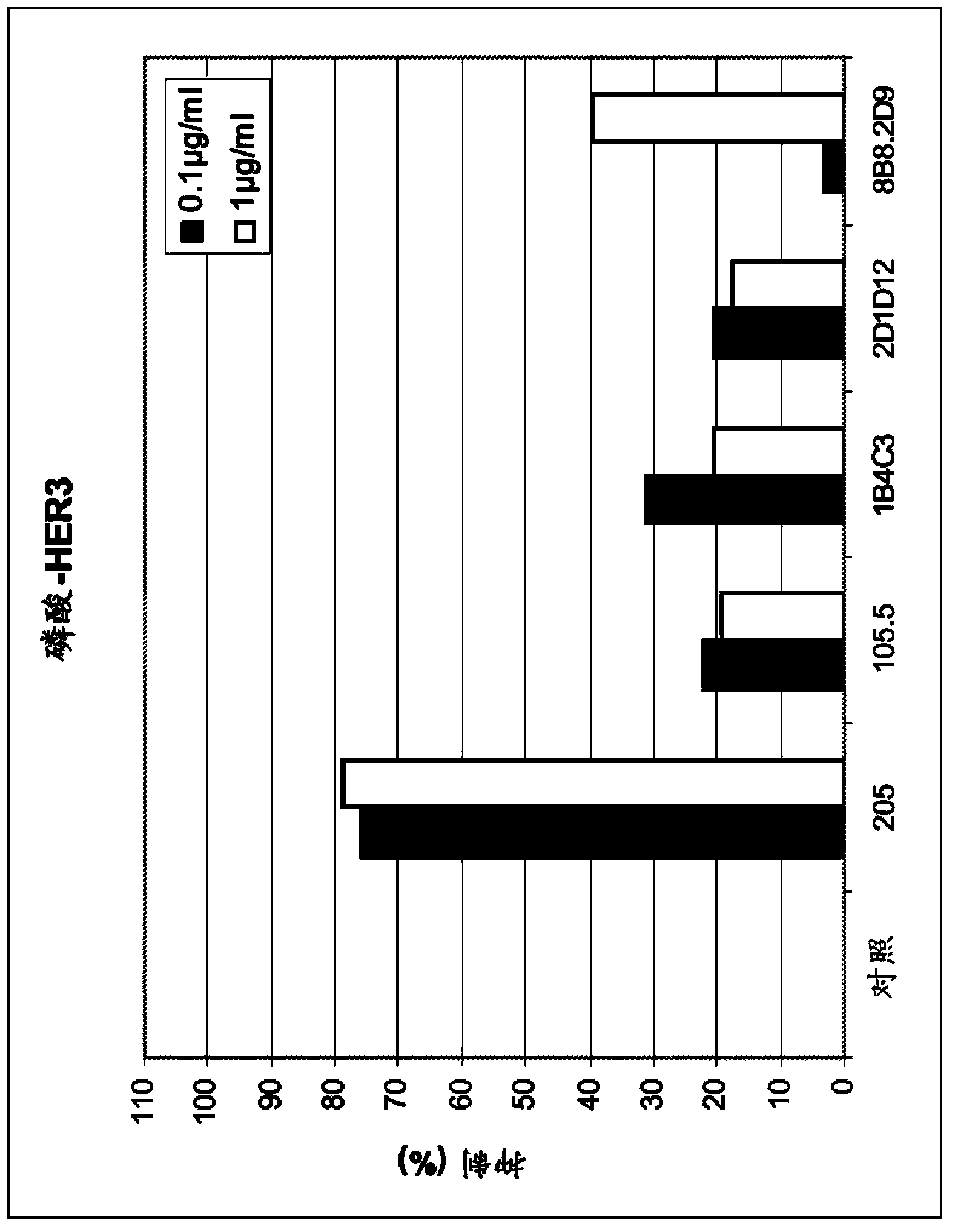
[0159] a) Inhibition of HER3 phosphorylation in MCF7, FaDu and Mel-Juso cells
[0160] Assays were performed in MCF7 and FaDu cells according to the following protocol: Cells were seeded at 500,000 cells / well in poly-D-lysine coated 6-well plates in RPMI1640 medium with 10% FCS. Incubate for 24 hours. Media was removed by aspiration and incubated overnight in 500 μl / well RPMI1640 with 0.5% FCS. Add 500 μl of antibody in RPMI 1640 with 0.5% FCS. Incubate for 1 hour. Add HRG-1b (final concentration 500 ng / ml) for 10 minutes. To lyse the cells, the medium was removed and 80 μl of ice-cold Triton-X-100 cell lysis buffer was added and incubated on ice for 5 minutes. After transferring the lysate into a 1.5 ml reaction tube and centrifuging at 14000 rpm for 15 minutes at 4°C, the supernatant was transferred into a fresh reaction tube. Samples containing equal amounts of protein in SDS loading buffer were resolved on SDS PAGE and blotted to nitrocellulose membranes by using semi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com