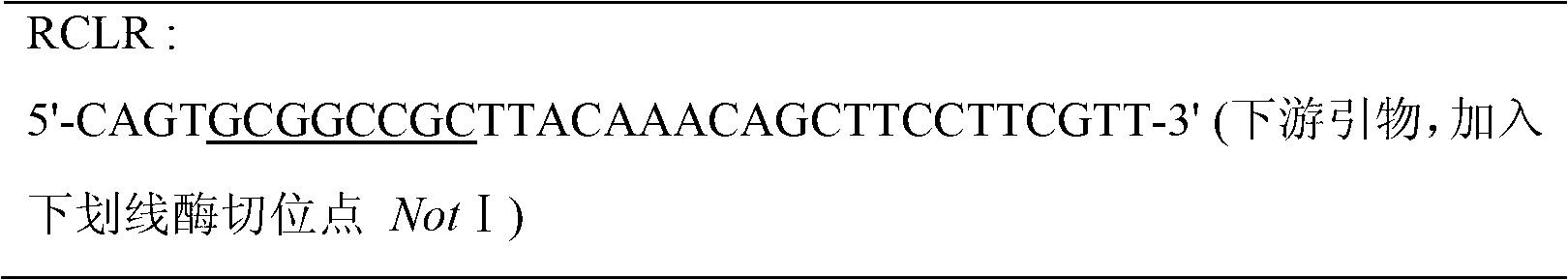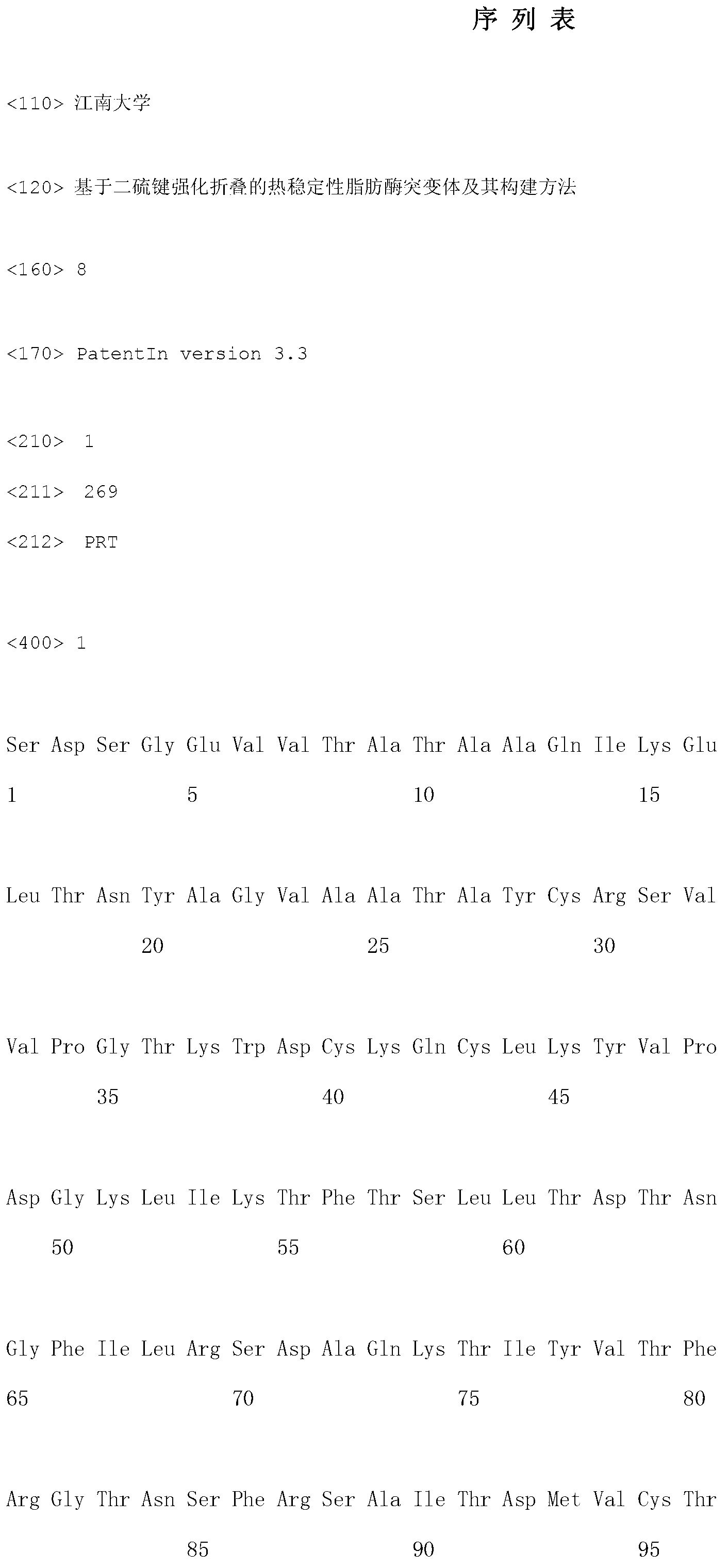Disulfide bond reinforced folding based lipase mutant with high heat stability and construction method thereof
A construction method and lipase technology, applied in the field of enzyme engineering, can solve the problem of low thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1, the design of disulfide bond mutation site
[0018] First, through the software SWISS-MODEL ( http: / / swissmodel.expasy.org / ) to establish the structure model of Rhizopus sinensis lipase, and use the software disulfide by design to predict the disulfide bonds that may be generated in the lipase structure. The prediction results indicated that two pairs of disulfide bonds may be formed in the lid hinge region of lipase, which are Phe95Cys / Phe214Cys and Asn84Cys / Gly266Cys respectively. NOTE: The lid of Rhizopus sinensis lipase and its hinge region are 82 GTNS FRSAIT DMVFT 96 , the lid region consists of 6 amino acids, underlined. Combined with the analysis of the structural model of the lipase, it was found that Asn84Cys / Gly266Cys is too close to the lid region, and the analysis may affect the opening of the lid, thereby causing a huge impact on the lipase activity. Therefore, a pair of disulfide bonds Phe95Cys / Phe214Cys, which is also located in the ...
Embodiment 2
[0019] Embodiment 2, construction of lipase mutant expression plasmid
[0020] The plasmid template pPIC9K-proRCL used in the present invention was constructed for previous studies [Wang Lele, Yu Xiaowei, Xu Yan, Cloning of Rhizopus chinensis leader peptide lipase gene and its expression in Pichia pastoris, High Technology Communications, ( 2009), 19(10):105], containing the lipase gene (proRCL) of Rhizopus chinensis CCTCC M201021. Two cysteine mutations Phe95Cys and Phe214Cys were introduced by overlap extension PCR, and the primers used were as follows:
[0021]
[0022]
[0023] PCR amplification conditions: 94°C for 3min; 94°C for 1min, 55°C for 1min, 72°C for 2min, 30 cycles; 72°C for 10min.
[0024] The amplified sequence was connected with pMD19-T simple, and the competent cells of large intestine were transformed by heat shock method. The positive strains were amplified and cultured, and the E. coli strains containing the plasmid pPIC9K were expanded at the s...
Embodiment 3
[0025] Example 3: Expression plasmid electrotransformation of Pichia pastoris, recombinant screening and molecular verification
[0026] After the positive strains were expanded and cultivated, the plasmids were extracted and digested with Sal Ⅰ to make them linear. The total amount was 5-10 μg for electroporation of Pichia pastoris competent. The electrorotator was set to a voltage of 1500V, a capacitance of 25μF, and a resistance of 200Ω. After electroporation, add 1 mL of sorbitol solution, revive at 30°C for 1 h, and spread a YPD plate with a G418 concentration of 0.25 mg / mL. The grown single colony is inoculated with YPD liquid medium and expanded to extract the genome. The genome is used as a template, and RCLF and RCLR are used as primers to carry out PCR reaction. If a band of about 1000bp is obtained, it is determined as a positive Pichia genetically engineered bacterium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com