Method for detecting impurity phenylhydrazine in edaravone
A detection method, the technology of edaravone, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of limiting the accuracy of phenylhydrazine, safety and ease of operation, etc.
Inactive Publication Date: 2012-12-26
CHENGDU BAIYU PHARMA CO LTD
View PDF0 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, these two methods have different defects, which limit the acc
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
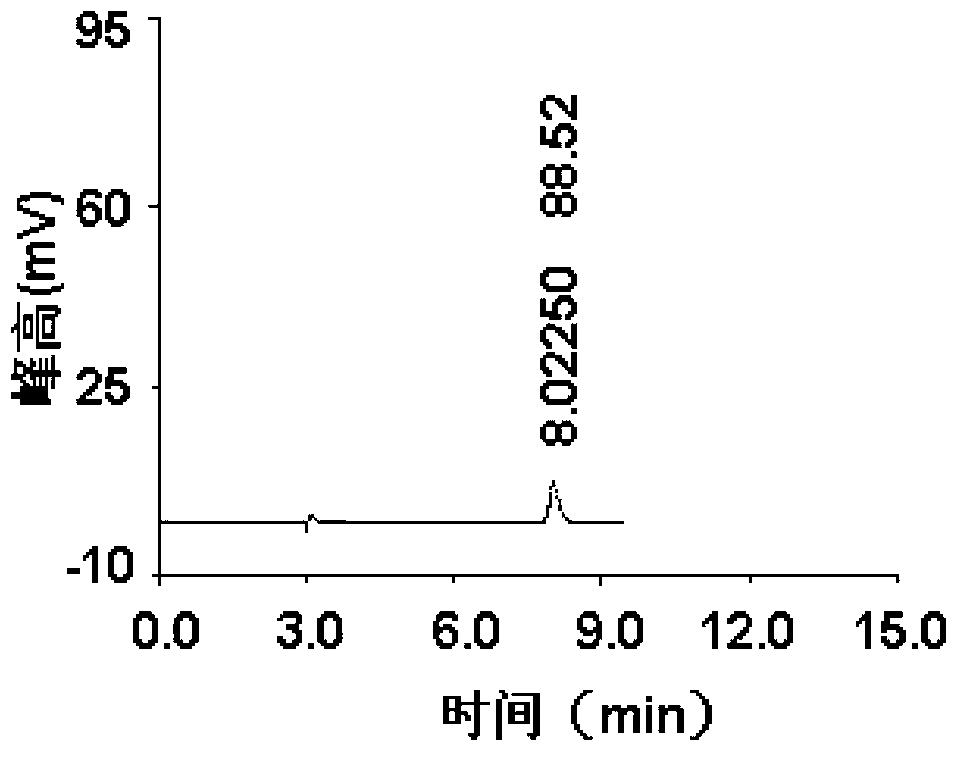
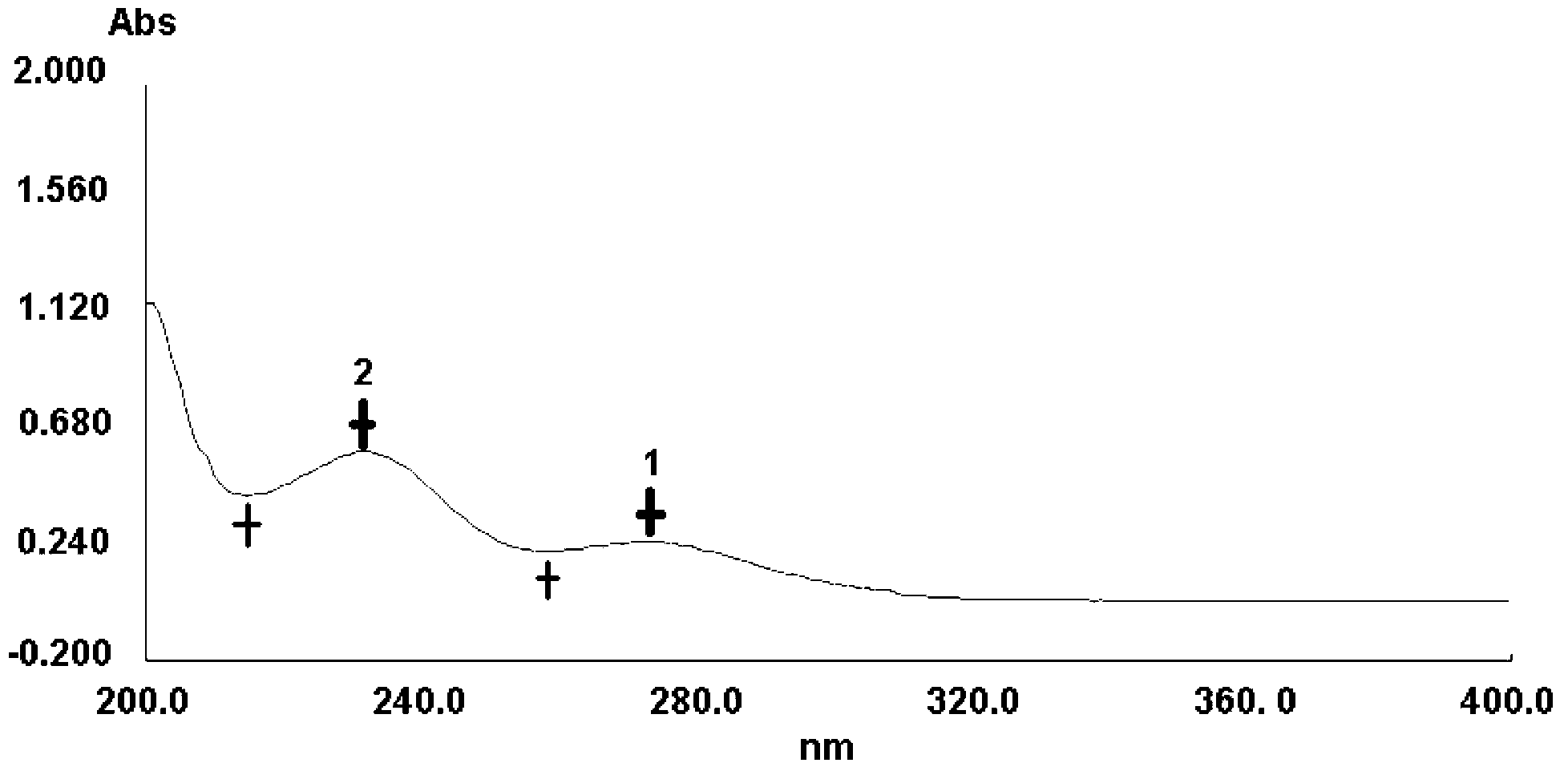
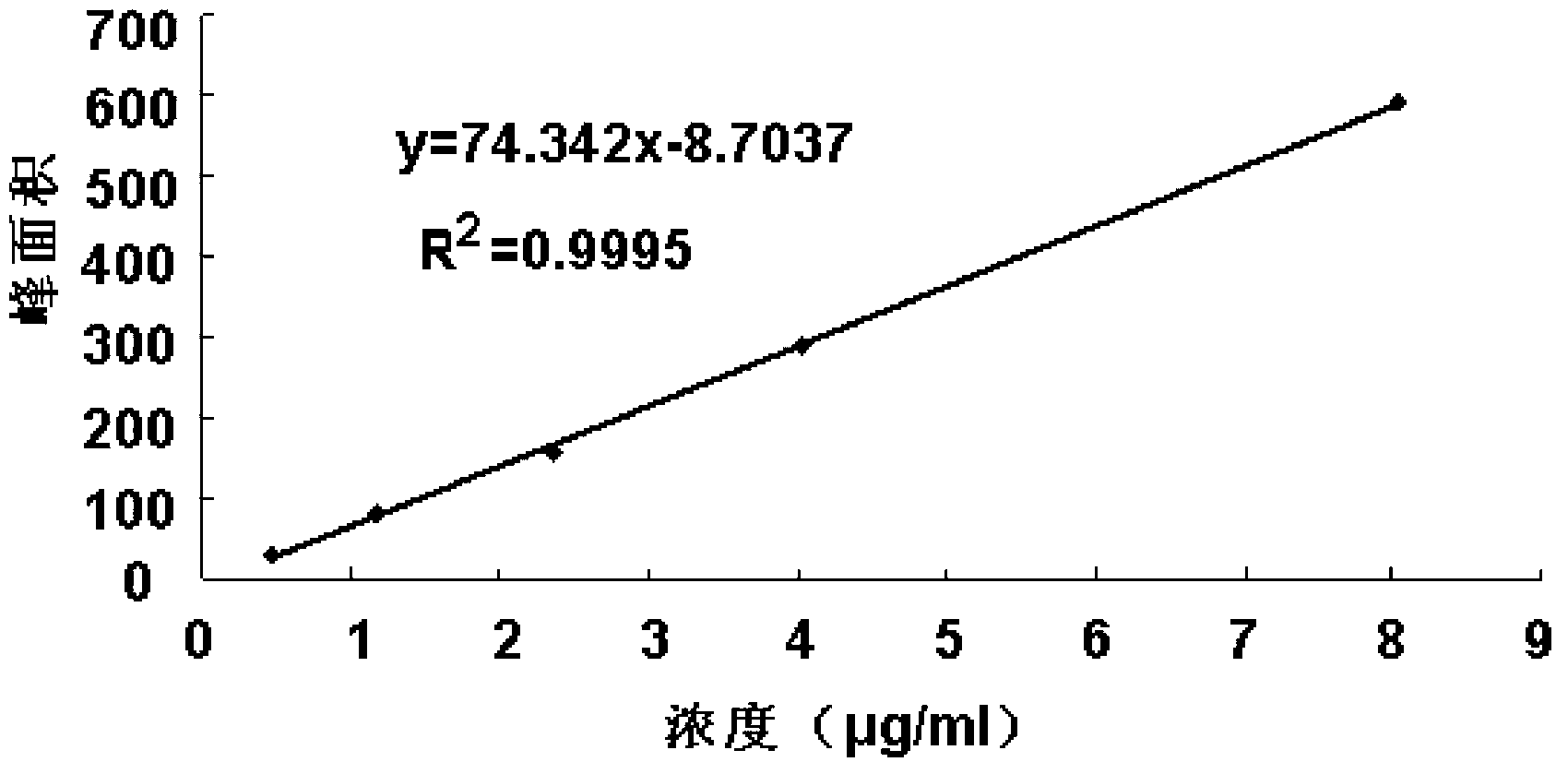
The invention belongs to the field of pharmaceutical analysis and specifically relates to a method for detecting impurity phenylhydrazine in edaravone. The invention aims to provide a conveniently operated, quick and accurate method for detecting the phenylhydrazine. The method comprises the following steps of: controlling a key HPLC (High Performance Liquid Chromatography) detecting condition by adopting liquid chromatogram detection, wherein for a moving phase, a volume percent of acetate buffer solution to carbinol is (70%-90%):(10%-30%) or the volume percent of acetate buffer solution to acetonitrile is (70%-90%):(10%-30%); the concentration of the acetate buffer solution is 0.04-0.06mol/L and pH value is 6.4-6.8; the detection wavelength is 226-238nm; the flow speed is 0.6-1.2ml/min; and the column temperature is 10-35 DEG C. The key detection parameters are controlled, so that the convenient, quick and accurate detecting purpose is achieved. The method provided by the invention has the advantages of convenience for operation accuracy and reliability in detecting result, stronger specificity, and shorter detection time because the main peak retention time is 9 minutes, and provides a brand new choice for detecting the impurity and controlling the product quality.
Description
technical field [0001] The invention belongs to the field of drug analysis, and in particular relates to a detection method for edaravone impurity phenylhydrazine. Background technique [0002] Edaravone is a brain protectant (free radical scavenger). The chemical name is 3-methyl-1-phenyl-2-pyrazolin-5-one; the molecular formula is: C 10 h 10 N 2O ; The chemical structure is as follows [0003] [0004] Clinical studies have shown that N-acetylaspartic acid (NAA) is a specific marker of surviving nerve cells, and its content decreases sharply in the early stage of cerebral infarction. The administration of edaravone to patients in the acute stage of cerebral infarction can inhibit the reduction of local cerebral blood flow around the infarction, and make the NAA content in the brain significantly higher than that of the glycerol control group on the 28th day after the onset. Mechanism studies suggest that Edaravone can scavenge free radicals and inhibit lipid peroxi...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): G01N30/88G01N30/06
Inventor 孙毅田阿娟陈明霞
Owner CHENGDU BAIYU PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com