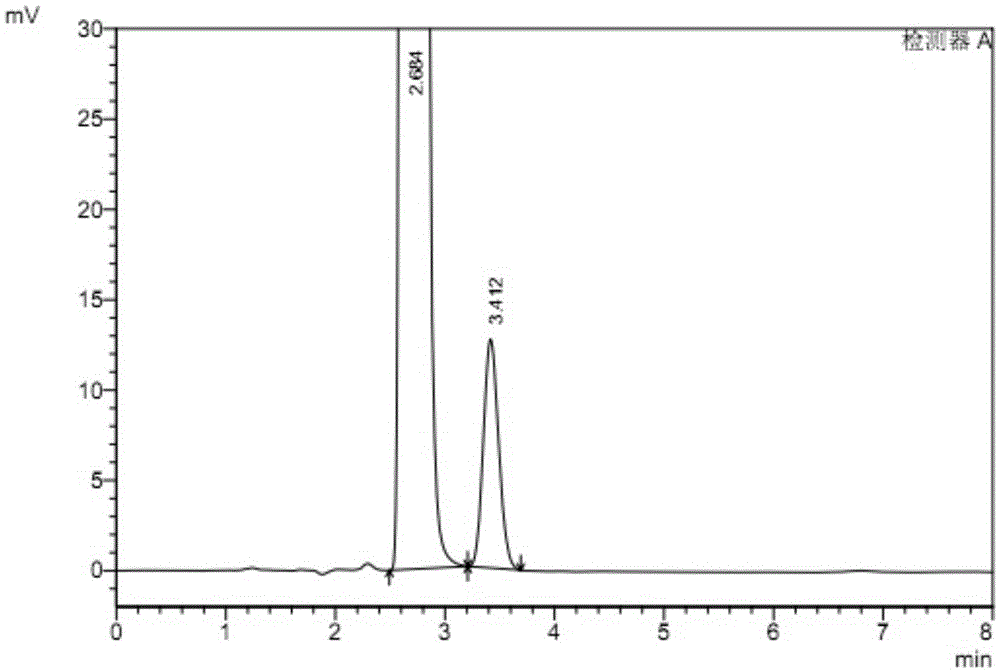
Patents
Literature
34results about How to "Symmetrical peak shape" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for detecting impurity phenylhydrazine in edaravone
InactiveCN102841170ASharp peakSymmetrical peak shapeComponent separationRetention timeColumn temperature
Owner:CHENGDU BAIYU PHARMA CO LTD
Method for measuring Jardiance and related substances of Jardiance through separation
ActiveCN106706768AWill not interfere with each otherStrong specificityComponent separationAlcoholSilanes
The invention discloses a method for measuring Jardiance and related substances of Jardiance through separation. According to the method, gradient elution are performed by adopting high performance liquid chromatography, utilizing octyl silane bonded silica gel filler or phenyl silane bonded silica gel filler as a chromatographic column and adopting a moving phase formed by a phosphoric acid solution, methyl alcohol and acetonitrile. With the adoption of the method, Jardiance and related substances of Jardiance can be separated very well, the detection is good in reproducibility, high in sensitivity and specificity and simple to operate, and control for quality and medication safety of Jardiance is facilitated.
Owner:CHONGQING PHARMA RES INST
Method for measuring content of tetrabutylammonium bromide in organic drug
ActiveCN104678026AWill not interfere with the determinationStrong specificityComponent separationTetramethylammonium hydroxidePhosphoric acid
The invention discloses a method for measuring residual amount of tetrabutylammonium bromide in an organic drug. The method is characterized in that the liquid chromatography is adopted, a reversed phase column is selected, and a mobile phase is formed by acetonitrile and a tetramethylammonium hydroxide and phosphoric acid solution for gradient elution. Compared with the prior art, the method provided by the invention achieves better separation of the tetrabutylammonium bromide from the organic drug, high test reproducibility, sensitivity and specificity, as well as simplicity and convenience in operation, and is conductive to the control of quality and usage safety for the organic drug.
Owner:CHONGQING PHARMA RES INST
Method for measuring patulin in fruit jam and vegetable paste
InactiveCN101776656AQualitatively accurateAccurate quantitative analysisComponent separationSodium bicarbonateGas chromatography–mass spectrometry
The invention discloses a method for rapidly measuring patulin in fruit jam and vegetable paste, comprising the following steps: weighing 2.00g of fruit jam and vegetable paste in a centrifuge tube, adding 0.5-3mL of water, shaking, adding 5mL of ethyl acetate-normal hexane solution (95+5, v / v), shaking, simultaneously adding 0.5-2g of sea sands, 4-8g of anhydrous sodium sulfate and 0.5-2g of sodium bicarbonate, shaking vigorously for 2min, and centrifuging for 3min; and weighing 4mL of supernate until ethyl acetate activates a carbon black columella, receipting eluent by a test tube in which glacial acetic acid is added, eluting by 3-7mL of ethyl acetate, and drying; dissolving 240 mu L of acetonitrile, pipetting 150 mu L of dissolution liquid into a sample bottle, adding 50 mu L of derivatization reagent, heating by microwave for 1-7min, cooling and then measuring by gas chromatography-mass spectrometry. The method is rapid, simple, sensitive, accurate, specific and durable in confirmation and quantification, and is widely applied to the measurement filed of patulin in fruit jam and vegetable paste.
Owner:新疆出入境检验检疫局检验检疫技术中心
Method for determining peramivir intermediate isomers by using high performance liquid chromatography
ActiveCN111983074AEfficient separationEasy to separateComponent separationChromatography columnHplc mass spectrometry
The invention relates to a method for determining peramivir intermediate isomers by using high performance liquid chromatography. The method is characterized in that an adopted chromatographic columnis a polysaccharide derivative coated chiral chromatographic column; the adopted mobile phase is a mixed solution of isopropanol and normal hexane, and isocratic elution is adopted in a high performance liquid chromatography system; in the mobile phase, the volume ratio of isopropanol to normal hexane is 10: 90 to 20: 80; the flow velocity of the mobile phase is 0.8 to 1.0 ml / min; an adopted detector is an ultraviolet detector, and the monitoring wavelength is 215 nm. The method overcomes the defects in the prior art, solves the problem of analytical determination of the peramivir intermediateisomer, can effectively control the contents of the target product and isomer impurities thereof, avoids the interference of the isomer impurities on the subsequent synthesis reaction, enhances the quality of the subsequent prepared peramivir, and ensures the medication safety. The invention provides an accurate and efficient detection method for determining the isomer of the peramivir intermediate.
Owner:苏州正济药业有限公司
High performance liquid chromatography (HPLC) detection method of Rivaroxaban
The invention discloses a high performance liquid chromatography (HPLC) detection method of Rivaroxaban. The method comprises the following steps: a. preparing a test solution; b. preparing a reference solution; c. respectively detecting the test solution and the reference solution by adopting HPLC, wherein the detection conditions are as follow: the stationary phase of a chromatographic column is silica gel which is coated with amylose-tri ((S)-alpha-methyl phenyl carbamate) on the surface, the mobile phase of the chromatographic column is acetonitrile-water, the flow velocity is 0.5-1.5ml / min, the column temperature is 10-40 DEG C, and the detection wave length is 200nm-300nm. After the detection method is used, the degree of separation of Rivaroxaban and Rivaroxaban R isomer is more than 3.0, and more theoretical plates are available, so that the detection result accuracy is effectively prevented from being influenced by the interference of all components; furthermore, the detection method has the advantages of being accurate and reliable in detection results, short in analysis time, low in cost, simple and convenient in operation, etc.
Owner:CHENGDU BAIYU PHARMA CO LTD
Detection method and application of acetic acid, propionic acid and butyric acid in traditional Chinese medicine injection
ActiveCN109682899AHigh sensitivityImprove accuracyComponent separationPropanoic acidInjection solution
The invention belongs to the technical field of traditional Chinese medicine injection detection, and particularly relates to a detection method and application of acetic acid, propionic acid and butyric acid in a traditional Chinese medicine injection. The detection method comprises the steps that the traditional Chinese medicine injection is diluted with an ethanol hydrochloride mixed solution,and acetic acid, propionic acid and butyric acid in the traditional Chinese medicine injection are detected through gas chromatography; the detection method is an internal standard method. By means ofthe method, the content of acetic acid, propionic acid and butyric acid in the traditional Chinese medicine injection can be detected simultaneously, quickly and accurately, the specificity is high,the reproducibility is good, and the defects of existing standards and other quantitative methods can be overcome. By means of the method, content determination of the three components in the Shuxuetong injection can be conducted quickly, accurately and simultaneously, and the method has great significance for improving the quality standard of the Shuxuetong injection.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
Method for testing ethylenediamine in lipoic acid injection medicine
ActiveCN109060973AEfficient detectionThe test result is accurateComponent separationEthylenediamineIon chromatography
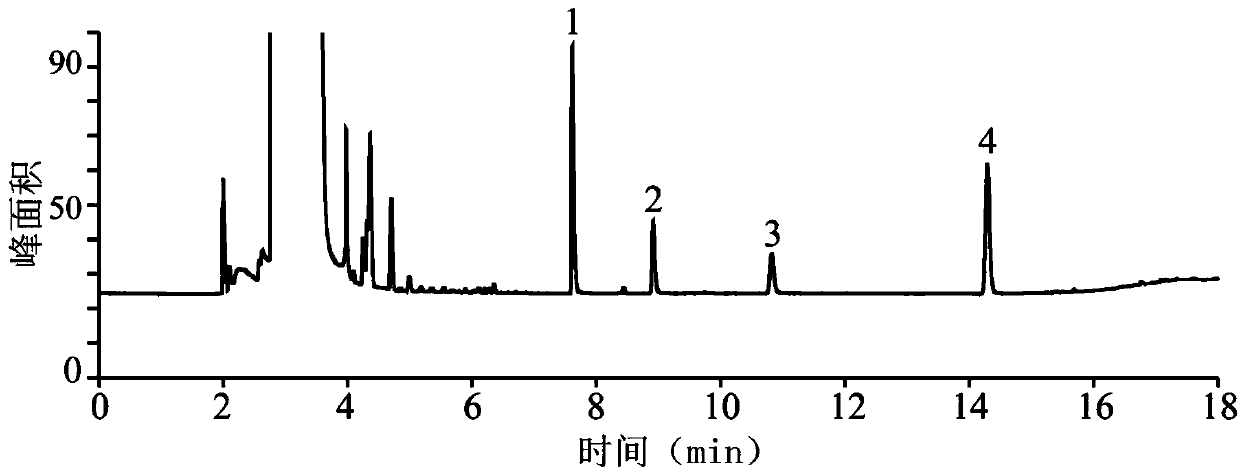
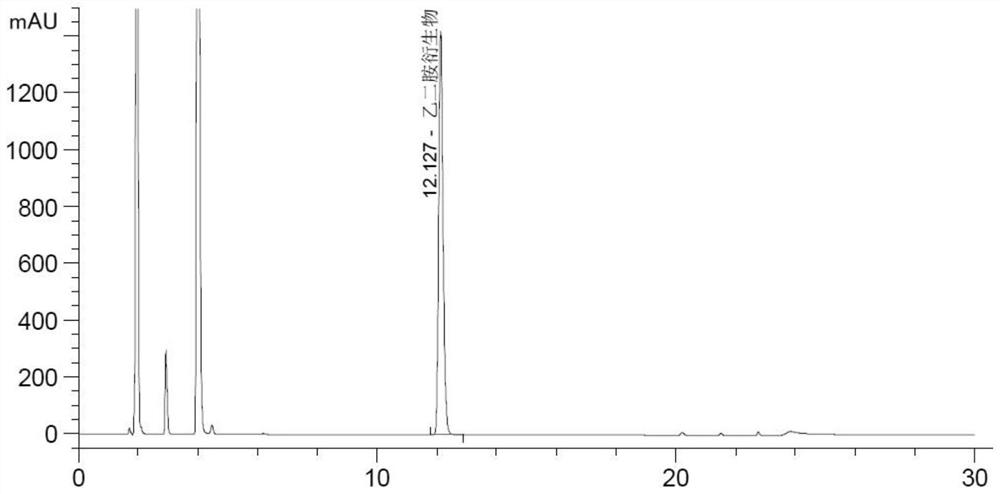
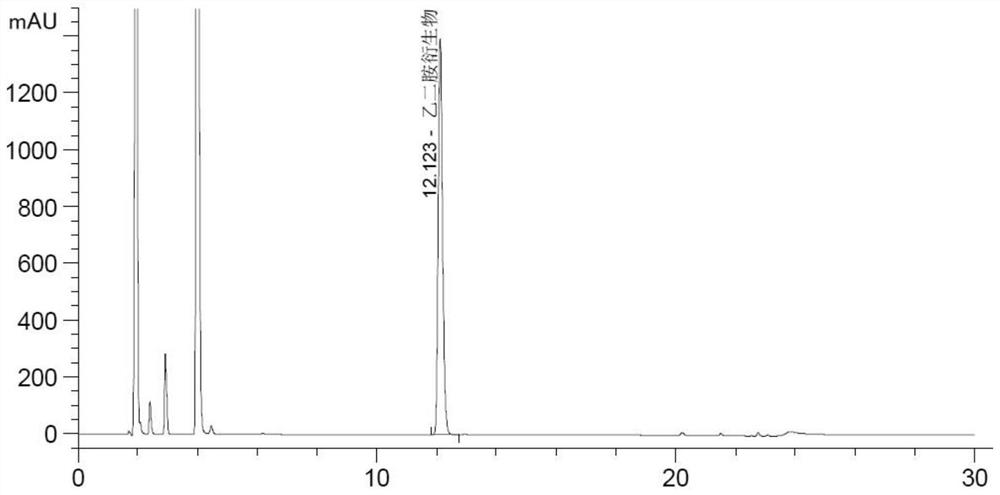
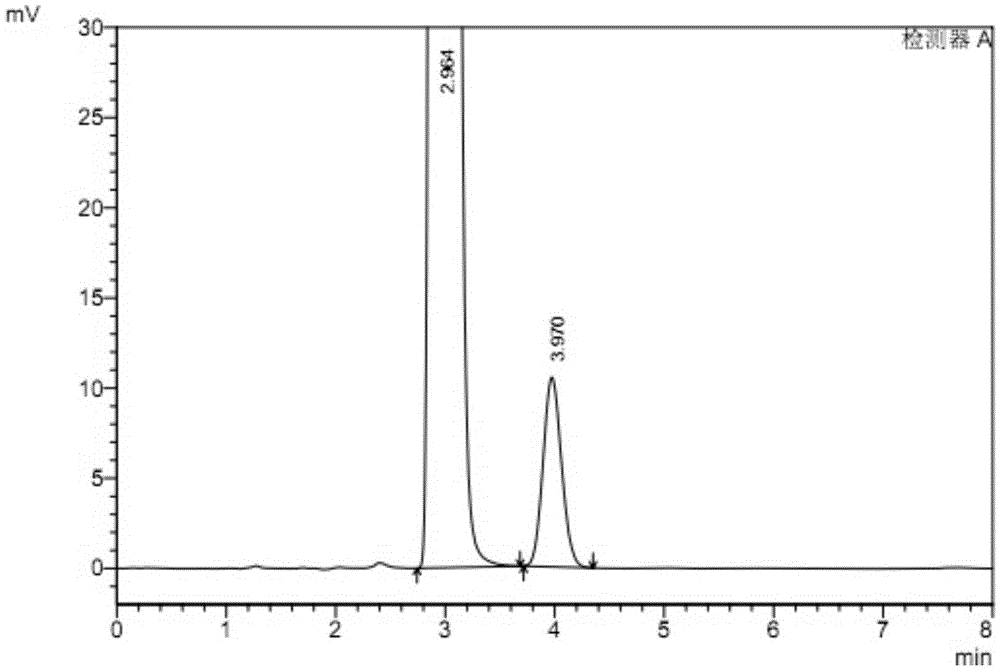
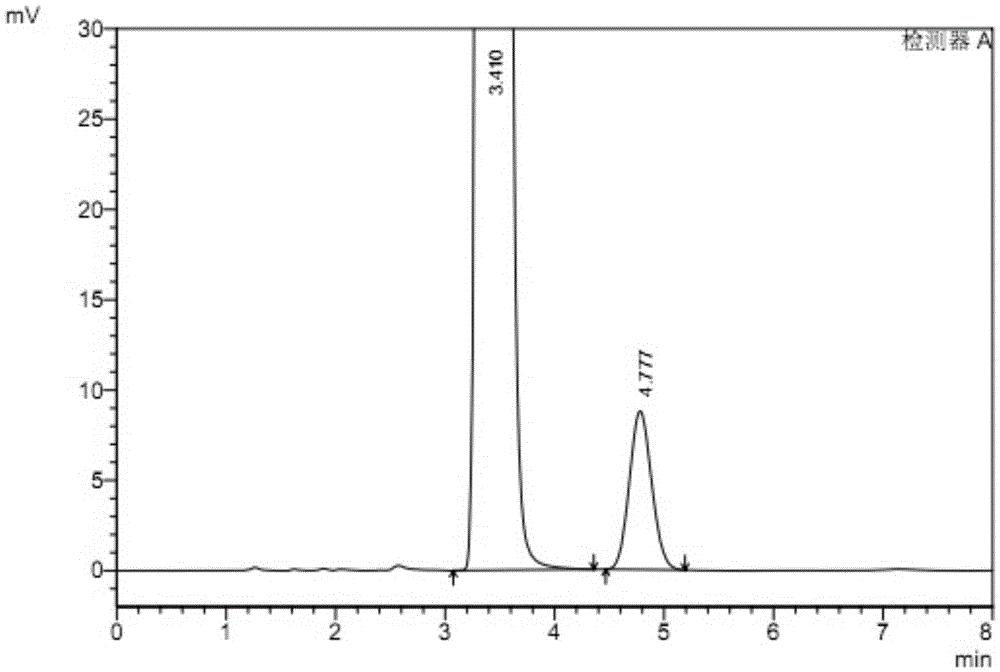
The invention relates to a method for testing ethylenediamine in lipoic acid injection medicine, which comprises the following steps: 1) preparing a reference solution of ethylenediamine; 2) preparinga test solution of zinc sulfate injection; 3) using the ion chromatography method to separately detect the reference solution and the test solution, and calculate the ethylenediamine dose. The conditions for ion chromatography include the following: in chromatography column the stationary phase is a cation exchange resin or a chemically bonded ion exchanger; the mobile phase includes the methanesulfonic acid in mobile phase A and the methanol or acetonitrile in mobile phase B; the volume ratio of mobile phase A to mobile phase B is 90-98:10-2. The ion chromatography method is effective in testing ethylenediamine. The result is accurate, with smooth baseline, symmetrical peak shapes, high stability, good repeatability and high sensitivity.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Detection method of navy blue dye in textile
InactiveCN102507759AReliable resultsHigh sensitivityComponent separationMaterial analysis by electric/magnetic meansPeak areaIon pairs
The invention discloses a detection method of navy blue dye in textile, which comprises preparing a to-be-detected sample solution; preparing a standard solution; injecting gradient standard solutions into a liquid chromatography-tandem mass spectrometer, determining peak positions of the navy blue dye by a negative ion multiple reaction monitoring (MRM) mode, recording the peak area of a quantificational ion pair, and working out a standard curve equation with the concentration as x coordinate and the peak area as y coordinate; and determining the navy blue dye in a supernatant obtained from the first step and its peak area in the same way, and calculating according to the standard curve equation to obtain the content of the navy blue dye in the sample. The method can rapidly, accurately, qualitatively and quantitatively detect the navy blue dye in the textile.
Owner:THE INSPECTION & QUARANTINE TECH CENT ZHEJIANG ENTRY EXIT INSPECTION & QUARANTINE BUREAU
Method for detecting content of ethylenediamine in medicine by HPLC
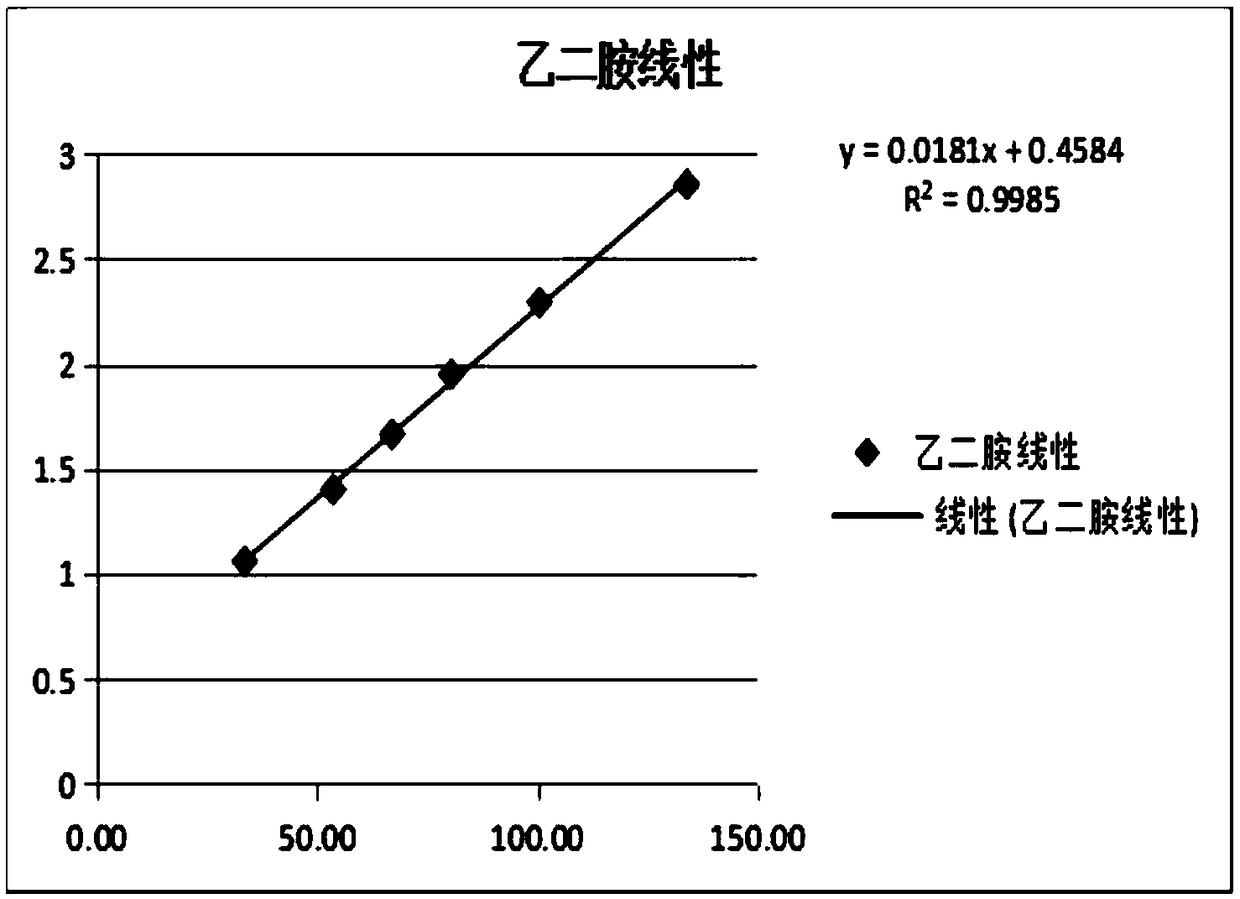
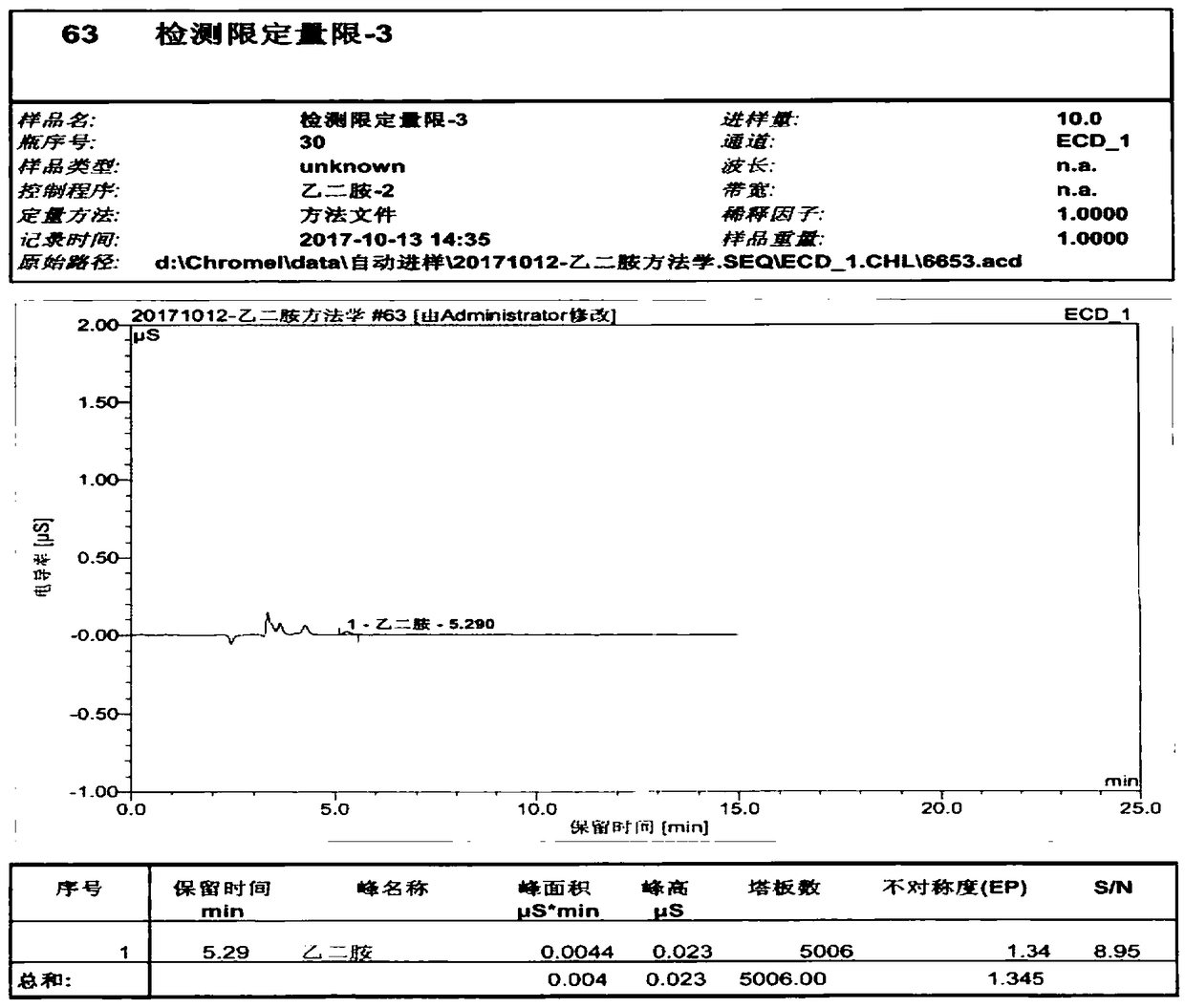
The invention particularly relates to a method for detecting a content of ethylenediamine in medicine by HPLC. The method is mainly realized by performing a derivatization reaction on ethylenediaminewithout ultraviolet absorption to obtain a derivative product with ultraviolet absorption. According to the method, the content of ethylenediamine is detected by adopting high performance liquid chromatography, and the method is accurate in detection result, flat in baseline, symmetrical in peak shape, high in stability, good in repeatability, high in separation degree, low in detection limit, high in sensitivity and free of damage to instruments.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
A method of high -efficiency liquid color spectrum detection method of Lidiastan
The invention discloses a high performance liquid chromatography (HPLC) detection method of Rivaroxaban. The method comprises the following steps: a. preparing a test solution; b. preparing a reference solution; c. respectively detecting the test solution and the reference solution by adopting HPLC, wherein the detection conditions are as follow: the stationary phase of a chromatographic column is silica gel which is coated with amylose-tri ((S)-alpha-methyl phenyl carbamate) on the surface, the mobile phase of the chromatographic column is acetonitrile-water, the flow velocity is 0.5-1.5ml / min, the column temperature is 10-40 DEG C, and the detection wave length is 200nm-300nm. After the detection method is used, the degree of separation of Rivaroxaban and Rivaroxaban R isomer is more than 3.0, and more theoretical plates are available, so that the detection result accuracy is effectively prevented from being influenced by the interference of all components; furthermore, the detection method has the advantages of being accurate and reliable in detection results, short in analysis time, low in cost, simple and convenient in operation, etc.
Owner:CHENGDU BAIYU PHARMA CO LTD
Detection on content of lincomycin in feeds through liquid chromatography-mass spectrography method
InactiveCN108037204AEasy to separateSymmetrical peak shapeComponent separationMass Spectrometry-Mass SpectrometryAntibiotic Y
The invention discloses a method of detecting the content of lincomycin hydrochloride in a feed through a high performance liquid chromatography method. A waters 2695 high performance liquid chromatograph is employed with a C18 chromatographic column; a mobile phase includes a 0.05 mol / L borax solution and methanol according to ratio of 55:45; flow rate is 1.0 ml / min; detection wavelength is 214 nm. The method has good separation between a main peak and adjacent peaks, in the range of 14.7-492 [mu]g / ml, the lincomycin hydrochloride can form good linearity, R2 = 0.9987 (n=6). The method is quick, sensitive and reliable, can be used for content detection of the lincomycin hydrochloride in a feed additive. The LC-MS / MS method can supply structural information of a to-be-detected substance; the method is high in sensitivity and selectivity and is an excellent method of detecting various antibiotics. The method is quick, sensitive, reliable and accurate.
Owner:吉林出入境检验检疫局检验检疫技术中心
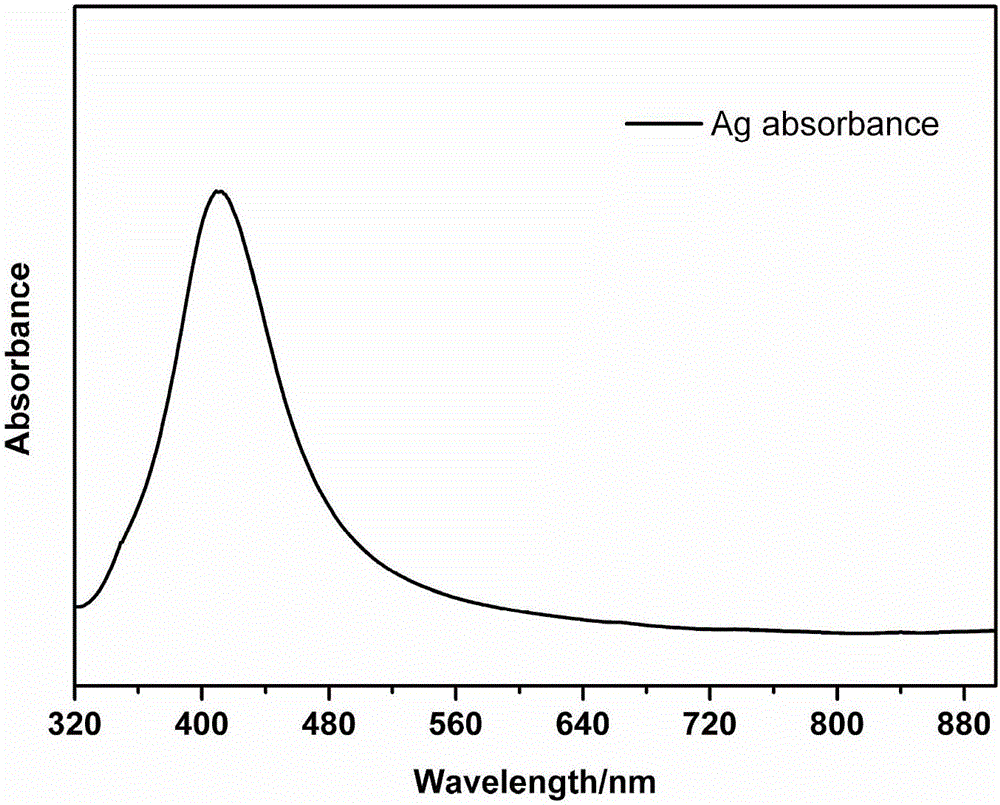
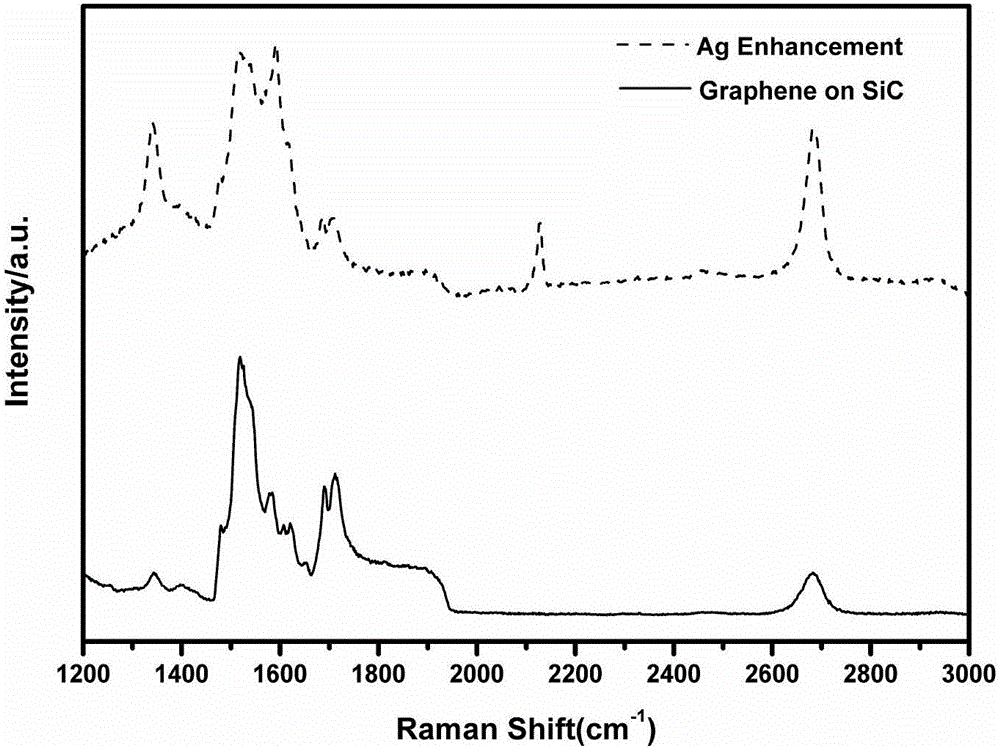
Method for characterization of graphene epitaxially grown on SiC based on Ag-particle Raman enhancement effect
The invention relates to a method for characterization of graphene epitaxially grown on SiC based on Ag-particle Raman enhancement effect. The method comprises the following steps: uniformly spraying Ag particles onto a quasi-freestanding graphene wafer at first, and then carrying out testing by using microscopic Raman spectroscopy so as to learn about the situation of bonding of a Si-H bond. According to the invention, the intercalation degree of hydrogen atoms can be directly determined by directly characterizing the Si-H bond in virtue of the Ag-particle Raman enhancement effect; influence of experimental conditions on hydrogen intercalation effect is visually fed back by analyzing the proportion of the Si-H bond in a testing area; and during visual characterization of the Si-H bond, the characteristic G peak, 2D peak and defect D peak of graphene are enhanced to certain extents, which greatly helps further accurate analysis of the structure and the properties like defects of graphene.
Owner:SHANDONG UNIV
A kind of method for separation and determination of empagliflozin and related substances
ActiveCN106706768BWill not interfere with each otherStrong specificityComponent separationO-Phosphoric AcidEngineering
The invention discloses a method for separating and measuring empagliflozin and its related substances. The method adopts high performance liquid chromatography, uses octylsilane bonded silica gel packing or phenylsilane bonded silica gel packing as a chromatographic column, and uses Phosphoric acid solution, methanol and acetonitrile constitute the mobile phase for gradient elution. The method can well separate empagliflozin and its related substances, has good detection reproducibility, high sensitivity, strong specificity, and simple operation, and is helpful for controlling the quality and drug safety of empagliflozin.
Owner:CHONGQING PHARMA RES INST
Preparation method of quinolone medicine passing type solid-phase extraction column
PendingCN113426158AImprove adsorption capacityAchieve purificationIon-exchange process apparatusOrganic chemistryQuinoloneOrganic solvent
The invention relates to a preparation technology of a solid-phase extraction column, and aims to provide a preparation method of a quinolone medicine passing type solid-phase extraction column. The method comprises the following steps: mixing a spiral COFs composite material with a proper amount of pure organic solvent, and carrying out ultrasonic treatment until the materials are uniformly dispersed; filling the pretreated material into a solid-phase extraction column made of a polypropylene material, and blocking two ends by using sieve plates respectively; mixing a traditional solid-phase extraction adsorbent with a proper amount of pure organic solvent, and performing ultrasonic treatment until uniform dispersion; continuously filling the pretreated material into the solid-phase extraction column, and blocking the end parts by using sieve plates to serve as sample introduction ends; and cleaning the column with a pure organic solvent, blowing with inert gas to remove the residual organic solvent, and drying in vacuum to obtain the quinolone medicine passing type solid-phase extraction column. The method has the advantages of simple preparation process, strong food impurity interception capability, good purification effect and the like. The matrix interference effect in the mass spectrum detection process can be obviously reduced, the mass spectrum detection response signal is effectively improved, and the sensitivity is effectively improved.
Owner:浙江方圆检测集团股份有限公司
Separation and detection method of related substances in ophthalmic gel
ActiveCN107356690BEnsure safetyQuality assuranceComponent separationSilica gelPharmaceutical Substances
Belonging to the field of pharmaceutical analysis, the invention relates to a separation and detection method for related substances in an ophthalmic gel. The method includes: preparing a to-be-detected solution, performing sample introduction, and conducting separation under the following conditions that: the chromatographic column is octadecyl silane bonded silica gel, the mobile phase is an acetonitrile-buffer solution and acetonitrile, the volume content of the mobile phase B is: 0% at 0-18min; 0-30% at 18-25min; 30% at 25-39min; 30-0% at 39-40min; and 0% at 40-50min; and conducting detection. The method can effectively separate related substances in the to-be-detected material. The control solutions of the related substances are prepared, the same method is adopted for high performance liquid chromatographic analysis, the peak area is read, and the content of each substance in the to-be-detected material is calculated. The method can rapidly and accurately determine the content of related substances in the to-be-detected material simultaneously, and is convenient for control of the product quality.
Owner:湖北远大天天明制药有限公司
A kind of method that liquid chromatography detects solid thiram potassium purity
ActiveCN108760961BSimple and fast operationEasy to separateComponent separationFluid phasePhysical chemistry
The invention provides a method for detecting solid potassium dimethyldithiocarbamate purity by a liquid phase chromatography method. According to the method, mobile phases are composed of a mobile phase a, a mobile phase b and a mobile phase c; the mobile phase c is an alkali organic amine compound. Derivation or preprocessing is not needed before liquid phase analysis is conducted on a sample, and sample analysis is directly carried out after dissolving and deaeration are conducted; in an obtained liquid phase chromatogram, all impurity peaks in the sample are detected, the separation effectbetween a main peak and the impurity peaks is good, the peak value is normal, the peak shape of the main peak is symmetric, smearing does not exist, and the detection time is short.
Owner:青岛中科荣达新材料有限公司
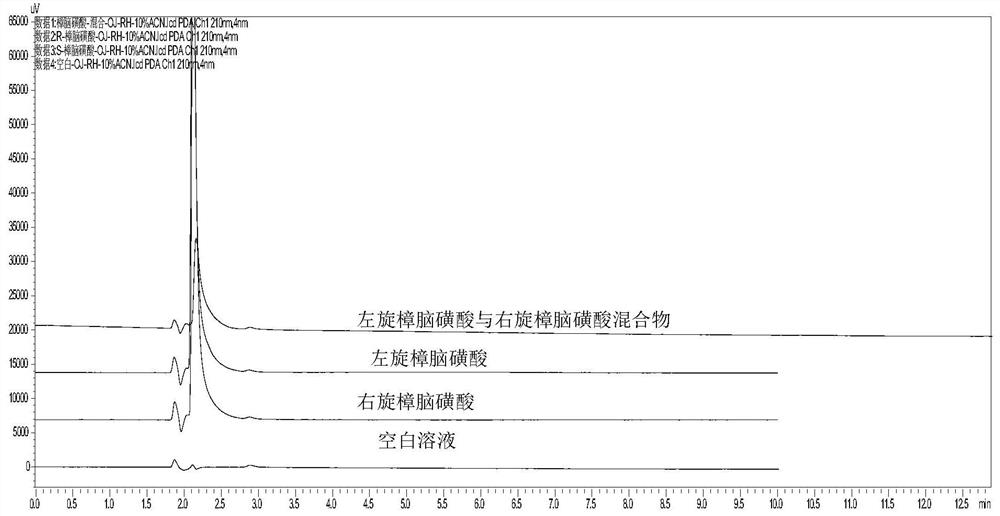
A kind of isomer detection method of camphorsulfonic acid or its salt
ActiveCN108362793BBaseline smoothSymmetrical peak shapeComponent separationAcetic acidTheoretical plate
The invention discloses a method for detecting isomers of camphorsulfonic acid or its salts. The detection method uses high performance liquid chromatography to qualitatively or quantitatively detect the isomers of camphorsulfonic acid or its salts. The detection conditions include: Chromatographic column: ion exchange column; detection wavelength: 210~400nm; mobile phase: organic phase containing acid and diethylamine, the acid is formic acid or acetic acid, and the detection method also includes the following steps: (1) preparing the Test solution, reference substance solution; (2) need test solution, reference substance solution sample injection detection respectively; Described need test solution is the isomer solution of camphorsulfonic acid or its salt, preferably L-camphorsulfonic acid solution or / and D-camphorsulfonic acid solution, more preferably L-camphorsulfonic acid solution. Adopting the method of the invention has good separation degree and high theoretical plate number.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
Preparation method of grafted high-capacity dendrimer ion chromatography stationary phase packing
InactiveCN104941611BSimple preparation processImprove stabilityOther chemical processesDendrimerGlycidyl methacrylate
Owner:ZHEJIANG UNIV
Method for measuring hydrogen sulfide content in desulfurized amine
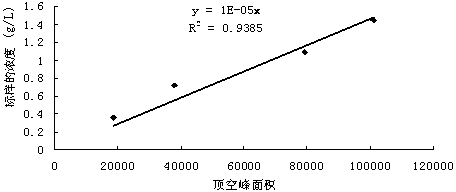
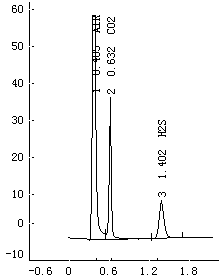
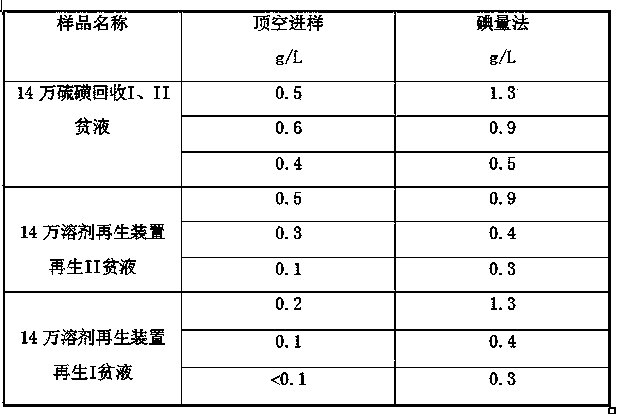
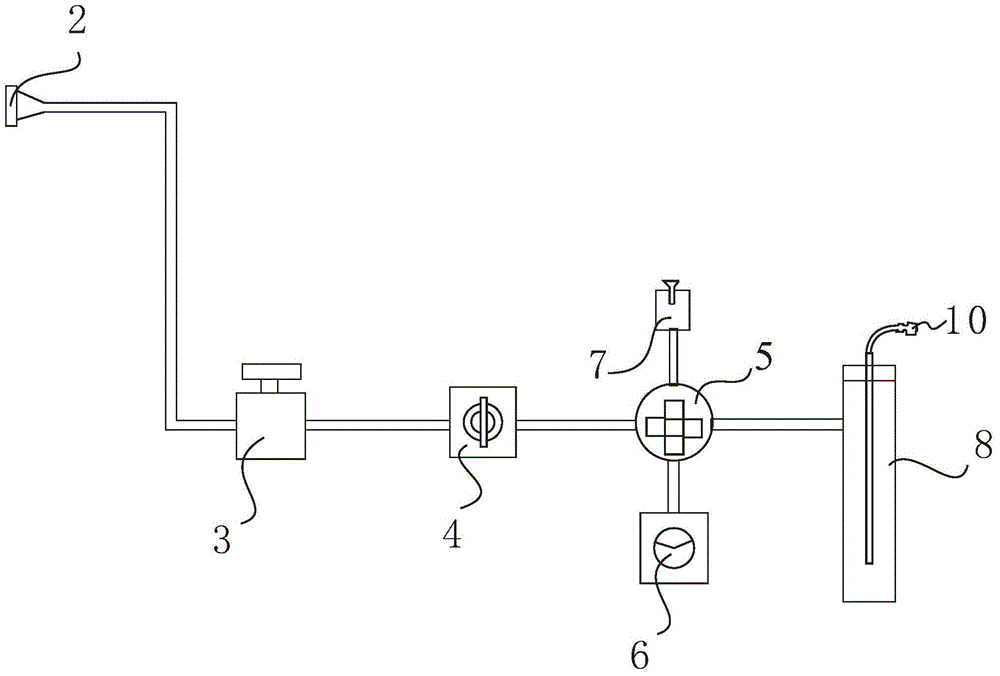
ActiveCN102507779BSymmetrical peak shapeAccurate measurementComponent separationInjection portGas phase
The invention relates to a method for measuring hydrogen sulfide content in desulfurized amine, which is used for measuring the hydrogen sulfide content in the desulfurized amine for refining and chemical enterprises. The method comprises the following steps of: adding a sample into a headspace bottle, sealing, adding dilute acid, shaking uniformly, and reacting the dilute acid with the sample to separate out hydrogen sulfide; standing, cooling to room temperature, performing chromatographic analysis on a headspace gas-phase component, and detecting by using a thermal conductivity cell detector; and calculating the hydrogen sulfide content in a barren solution in the desulfurized amine by using hydrogen sulfide standard gas configuration and a standard liquid calibrator according to the hydrogen sulfide content in a standard liquid, a peak area of the headspace gas-phase component hydrogen sulfide, and a peak area of a headspace gas-phase component hydrogen sulfide of the sample. By the method, the analysis is quick, and a chromatographic column and an injection port are prevented from being polluted by the sample and impurities, so that a chromatogram baseline is stable, peak shapes of hydrogen sulfide are symmetric, the interference of other substances on analysis is eliminated, and the analysis accuracy is improved.
Owner:CHINA PETROLEUM & CHEM CORP
Carrier coating device and coating method for capillary chromatography substrate
InactiveCN104865329BIncrease the maximum use temperatureIncrease success rateComponent separationExhaust valveInlet valve
The invention discloses a carrier coating device of a capillary chromatography base pipe. The carrier coating device comprises an air inlet, an air inlet valve, a flow speed control valve and a copper pressure-resisting pipe which are in sealed connection in sequence through pipelines, wherein the pipeline between the flow speed control valve and the copper pressure-resisting pipe is further connected with an exhausting valve and a pressure gauge; the copper pressure-resisting pipe is provided with a cavity and a sealing cover; and the sealing cover is provided with a leading-in pipe for inserting the capillary chromatography base pipe. The invention further provides a method for coating the capillary chromatography base pipe with a carrier, so that ion liquid coating is well performed, and the coating effect of the ion liquid with middle and strong polarities is enhanced, and furthermore, the separation capability of fixed phases for some complicated samples is improved.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Method for water-phase rapid synthesis of CdTe nano crystal at warm condition
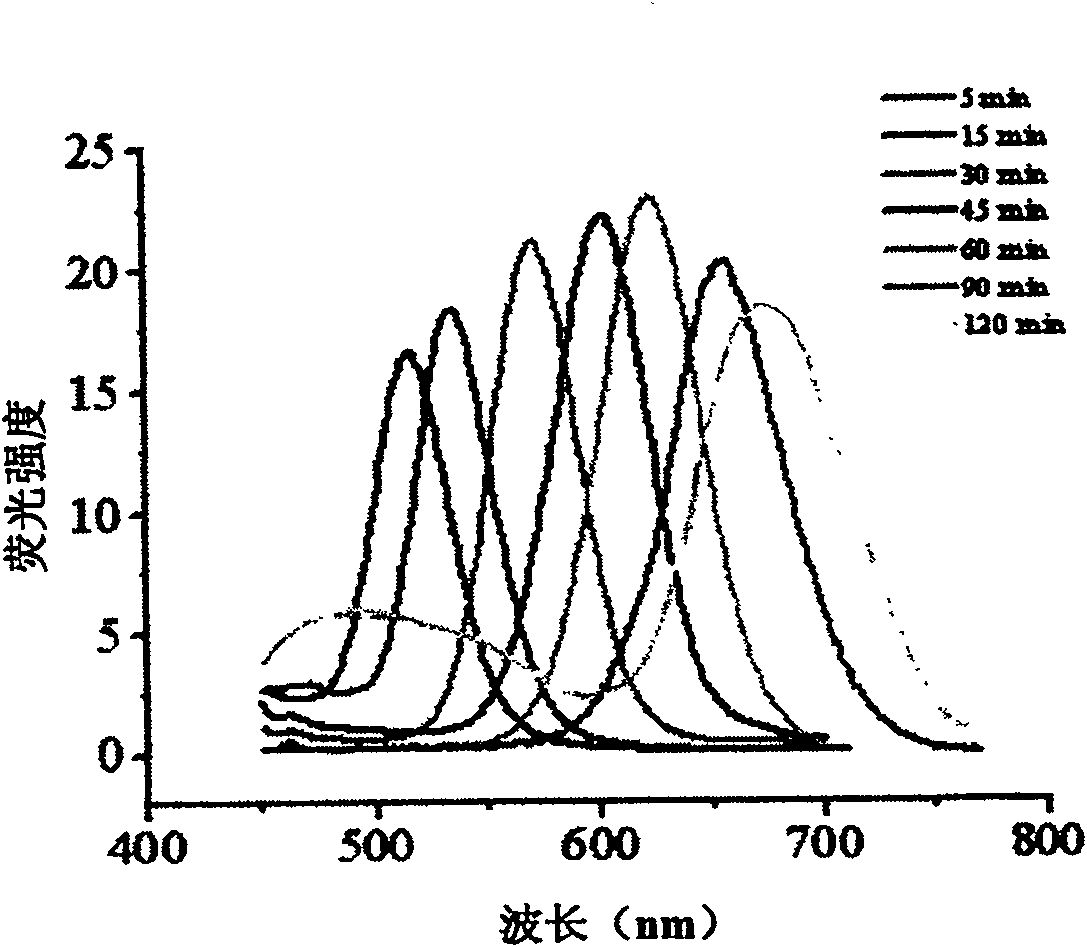
InactiveCN100554532CSynthesis fastHigh yieldPolycrystalline material growthSingle crystal growth detailsQuantum yieldBiocompatibility Testing
The invention relates to a method for synthesizing water-soluble CdTe nanocrystals under mild conditions. Inorganic tellurium powder and cadmium salt or their hydrates are used as reactants, glutathione is used as a stabilizer, the synthesis temperature is 60-120°C in water phase, under mild conditions, the pH of the solution is 7.0-12.0, and the reaction time is In an open system, CdTe nanocrystals can be synthesized safely and rapidly within 5 minutes to 2 hours. The fluorescent color changes from green through various intermediate colors such as yellow and orange, and finally becomes red. The CdTe nanocrystals prepared by the method have good size controllability, good monodispersity, narrow and symmetrical fluorescence emission peaks; good biocompatibility; good repeatability of the method; quantum yield can reach 40%; stability it is good. The CdTe nanocrystal synthesized by the invention can be widely used in the research field of biological fluorescence labeling.
Owner:JILIN UNIV
Method for separating and determining two genotoxic impurities in flurbiprofen axetil
PendingCN113008999AAccurate quality controlAccurate measurementComponent separationO-Phosphoric AcidSilica gel
The invention discloses a method for separating and determining genotoxic impurities vinyl acetate and benzene in flurbiprofen axetil. According to the method, liquid chromatography is adopted, an octadecyl silica gel chromatographic column is used as a stationary phase, acetonitrile-phosphoric acid-water is used as a mobile phase, vinyl acetate and benzene can be effectively separated, all impurities are good in peak shape, the column efficiency is high, specificity is good, and the quality of flurbiprofen axetil is well controlled.
Owner:YAOPHARMA CO LTD
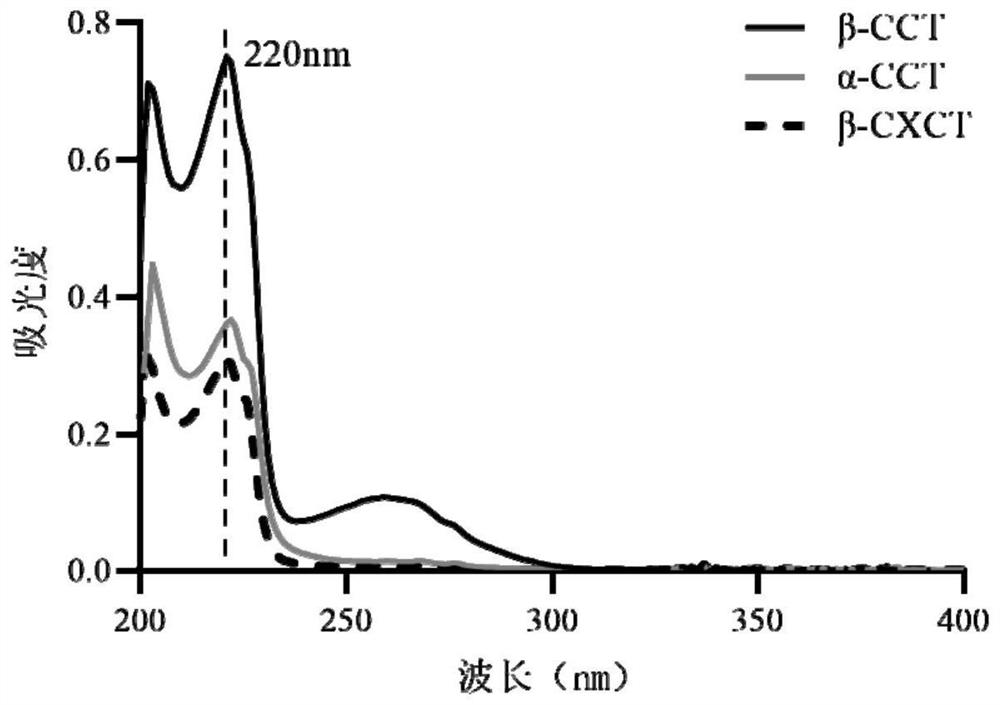
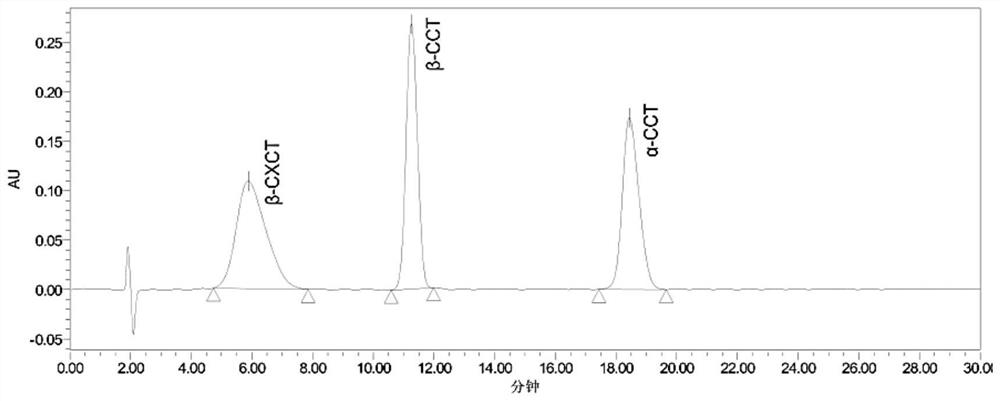
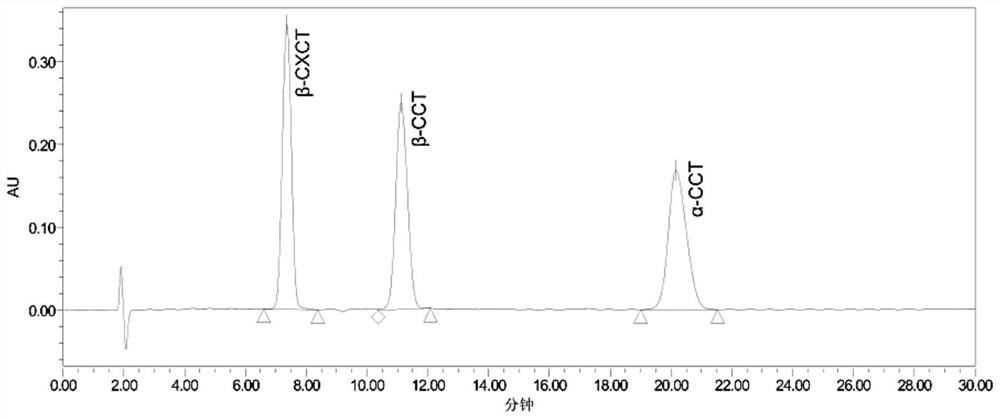
Method for determining 2beta-methyl-3beta-(4-chlorphenyl) tropine and related substances thereof
The invention provides a method for simultaneously determining 2beta-methyl-3beta-(4-chlorphenyl) tropane and related substances thereof, belongs to the field of pharmaceutical analysis, and overcomes the defects that beta-CCT and related substances thereof cannot be separated and the quality of beta-CCT cannot be detected in the conventional analysis method. According to the method for simultaneously determining the 2beta-methyl-3beta-(4-chlorphenyl) tropane and the related substances thereof, high performance liquid chromatography is adopted, a used chromatographic column is a C18 reversed-phase column, a mobile phase comprises methanol, water and an acidic additive, and the volume ratio of the methanol to the water to the acidic additive is (25-40): (60-75): (0.05-1.5); the detection method is ultraviolet detection; the related substances are 2 [alpha]-methyl ester-3 [beta]-(4-chlorphenyl) tropine alkane and 2 [beta]-carboxyl-3 [beta]-(4-chlorphenyl) tropine alkane. According to the method disclosed by the invention, the beta-CXCT, the beta-CCT and the alpha-CCT can be subjected to baseline separation under the same HPLC (High Performance Liquid Chromatography) condition.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
A kind of method for measuring tetrabutylammonium bromide content in organic medicine
ActiveCN104678026BWill not interfere with the determinationStrong specificityComponent separationTetramethylammonium hydroxidePhosphoric acid
The invention discloses a method for measuring residual amount of tetrabutylammonium bromide in an organic drug. The method is characterized in that the liquid chromatography is adopted, a reversed phase column is selected, and a mobile phase is formed by acetonitrile and a tetramethylammonium hydroxide and phosphoric acid solution for gradient elution. Compared with the prior art, the method provided by the invention achieves better separation of the tetrabutylammonium bromide from the organic drug, high test reproducibility, sensitivity and specificity, as well as simplicity and convenience in operation, and is conductive to the control of quality and usage safety for the organic drug.
Owner:CHONGQING PHARMA RES INST
A method for detecting ethylenediamine in lipoic acid injection
ActiveCN109060973BEfficient detectionThe test result is accurateComponent separationIon chromatographyMeth-
The present invention specifically relates to a method for detecting ethylenediamine in lipoic acid injection, comprising the following contents: 1) taking ethylenediamine to prepare a reference substance solution; 2) taking zinc sulfate injection to prepare a test solution; 3) using ion Chromatography detects reference substance solution and need testing solution respectively, calculates ethylenediamine content; Wherein the chromatographic condition of ion chromatography comprises the following content: Chromatographic column: stationary phase is cation exchange resin or chemically bonded ion exchanger; Mobile phase: mobile phase Phase A is methanesulfonic acid, mobile phase B is methanol or acetonitrile; the volume ratio of mobile phase A and mobile phase B is 90-98:10-2. The ion chromatography method of the invention is used to detect ethylenediamine, which can effectively detect ethylenediamine, and the detection result is accurate, the baseline is flat, the peak shape is symmetrical, the stability is high, the repeatability is good, and the sensitivity is high.
Owner:CHENGDU BRILLIANT PHARMA CO LTD
A kind of method for determining peramivir intermediate isomer by high performance liquid chromatography
ActiveCN111983074BEfficient separationStrong specificityComponent separationAlkaneChromatography column
Owner:苏州正济药业有限公司
High pH tolerance chromatographic filler and preparation method thereof
ActiveCN102247821BImprove featuresHigh column efficiencyOther chemical processesSilica gelSpherical form
The invention provides a high pH tolerance chromatographic filler and a preparation method thereof. The filler is prepared by using ultra-pure entire porous spherical silica gel as matrix, and coating a high pH tolerance inorganic / organic hybrid layer with the thickness of several nm through a reaction for surface modification in advance. The filler not only keeps the advantages of other silica-based fillers, but also overcomes the defects of narrow pH range of the traditional silica-based fillers, so as to broaden the PH range to 1-12.5. In the preparation of the filler, an inorganic / organichybrid polymer is prefabricated, then the inorganic / organic hybrid polymer is reacted with the silica gel matrix, and the hybrid layer is coated on the silica gel matrix.
Owner:月旭科技(上海)股份有限公司 +1
A kind of detection method and application of acetic acid, propionic acid and butyric acid in traditional Chinese medicine injection
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
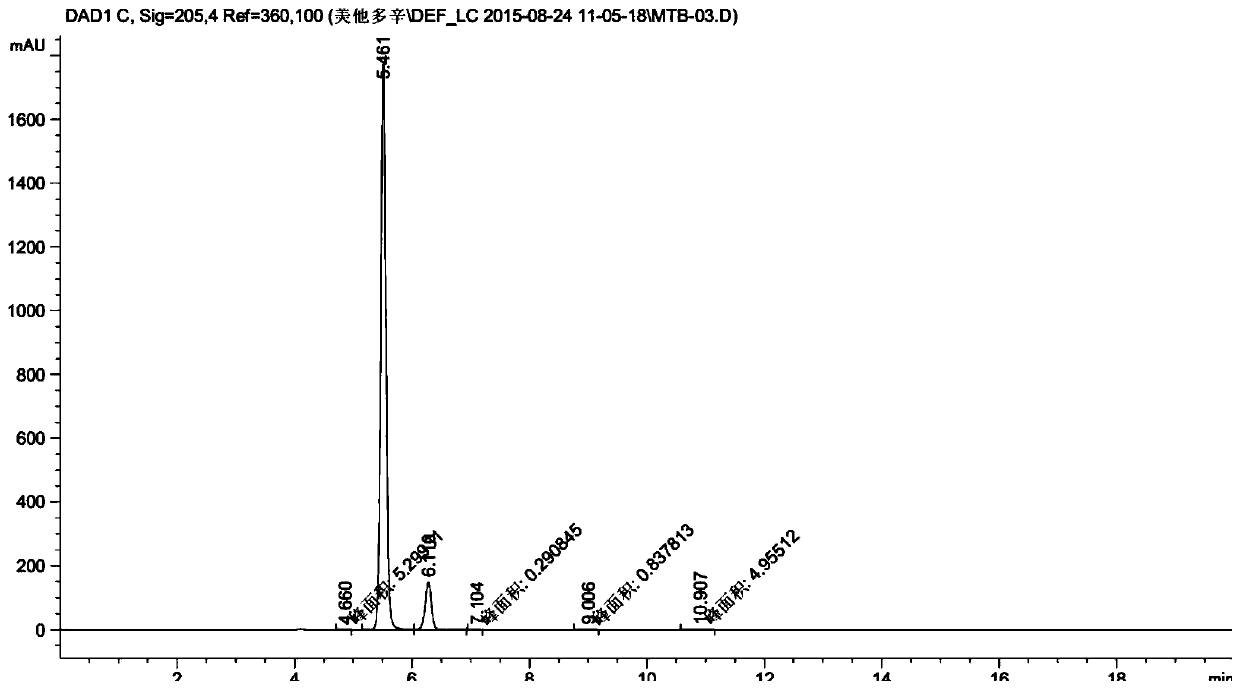
A method for the detection of related substances of metadoxine by high performance liquid chromatography
ActiveCN106855542BEasy to separateSharp peakComponent separationRetention timeHigh-performance Liquid Chromatography-UV
Belonging to the technical field of pharmaceutical analysis, the invention in particular relates to a method for detection of metadoxine related substances by high performance liquid chromatography. A metadoxine test solution and a reference solution are detected by high performance liquid chromatography respectively, chromatograms of the two are recorded, a peak area of vitamin B6 in the metadoxine test solution and a main peak area of the reference solution are acquired, and quantitative analysis is carried out on metadoxine related substances by external standard method. The reference solution is a pure metadoxine solution. The method provided by the invention has the advantages of high separation degree, moderate principal component retention time, i.e. short analysis time, and low cost.
Owner:SICHUAN TONGSHENG BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com