Method for measuring content of tetrabutylammonium bromide in organic drug
A technology of tetrabutylammonium bromide and organic drugs, applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve the problems of low penetration rate of instruments, unfriendly environment, poor specificity, etc., achieves strong specificity, avoids Long analysis time, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
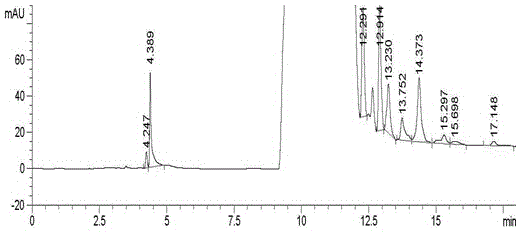
Embodiment 1
[0050] Instruments and Conditions
[0051] High performance liquid chromatography: Agilent 1260;
[0052] Chromatographic column: Octadecylsilane bonded silica gel as filler (AichromBond-AQ C18 4.6 mm×250mm, 5μm);
[0053] The mobile phase A is the aqueous phase: 0.005mol / L tetramethylammonium hydroxide solution, the pH value is adjusted to 3.0 with phosphoric acid, and the mobile phase B is the organic phase: acetonitrile.
[0054] Elute according to the gradient elution table (see Table 1) (after 5 minutes, it is the equilibrium system stage).
[0055]
[0056] Injection volume: 20μl;
[0057] Column temperature: 30°C;
[0058] Detection wavelength: 210nm.
[0059] Experimental procedure
[0060] Take about 400 mg of the crude drug canagliflozin, accurately weigh it, put it in a 10ml volumetric flask, add acetonitrile aqueous solution (acetonitrile:water=90:10v / v, the same below) to dissolve and dilute to the mark, shake well, and serve as Test solution. Take about...
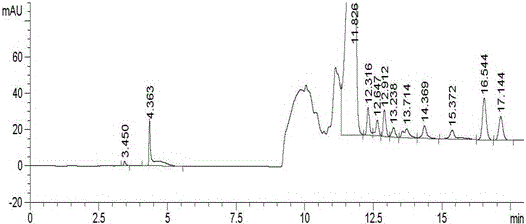
Embodiment 2
[0063] Instruments and Conditions
[0064] High performance liquid chromatography: Agilent 1260;
[0065] Chromatographic column: Octadecylsilane bonded silica gel as filler (AichromBond-AQ C18 4.6 mm×250mm, 5μm);
[0066] Mobile phase A: aqueous phase 0.005mol / L tetramethylammonium hydroxide solution, adjust the pH value to 3.0 with phosphoric acid, mobile phase B: acetonitrile;
[0067] Elute according to the gradient elution table (see Table 2) (after 5 min, it is the equilibrium system stage).
[0068]
[0069] Injection volume: 20μl;
[0070] Column temperature: 30°C
[0071] Detection wavelength: 210nm.
[0072] Experimental procedure
[0073] Take about 400mg of the crude drug of canagliflozin, weigh it accurately, put it in a 10ml volumetric flask, add an appropriate amount of tetrabutylammonium bromide solution accurately, add acetonitrile aqueous solution to dissolve and dilute to make tetrabutylammonium bromide in every 1ml 4 μg of the mixed solution, shake...
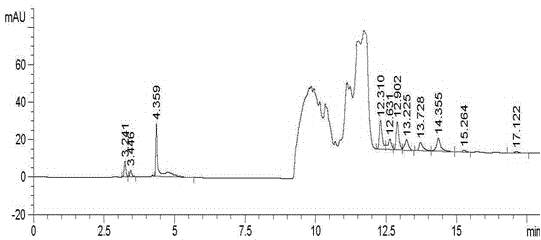
Embodiment 3
[0075] Instruments and Conditions
[0076] High performance liquid chromatography: Agilent 1260;
[0077] Chromatographic column: Octadecylsilane bonded silica gel as filler (AichromBond-AQ C18 4.6 mm×250mm, 5μm);
[0078] Mobile phase A: aqueous phase 0.005mol / L tetramethylammonium hydroxide solution, adjust the pH value to 2.0 with phosphoric acid, mobile phase B: acetonitrile;
[0079] Elute according to the gradient elution table (see Table 3) (after 5 min, it is the equilibrium system stage).
[0080]
[0081] Injection volume: 20μl;
[0082] Column temperature: 30°C.
[0083] Experimental procedure
[0084] Take about 10 mg of tetrabutylammonium bromide reference substance, accurately weigh it, put it in a 25ml volumetric flask, add acetonitrile aqueous solution to dissolve and dilute to the mark, shake well, and use it as a stock solution. Take 500mg of Empagliflozin crude drug, accurately weighed, put in a 10ml volumetric flask, accurately add 0.1ml of stock so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com