High performance liquid chromatography (HPLC) detection method of Rivaroxaban
A high-performance liquid chromatography and detection method technology, which is applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of long analysis time, low separation degree of rivaroxaban S isomer and R isomer, etc. Short analysis time, accurate and reliable detection results, and sharp peaks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
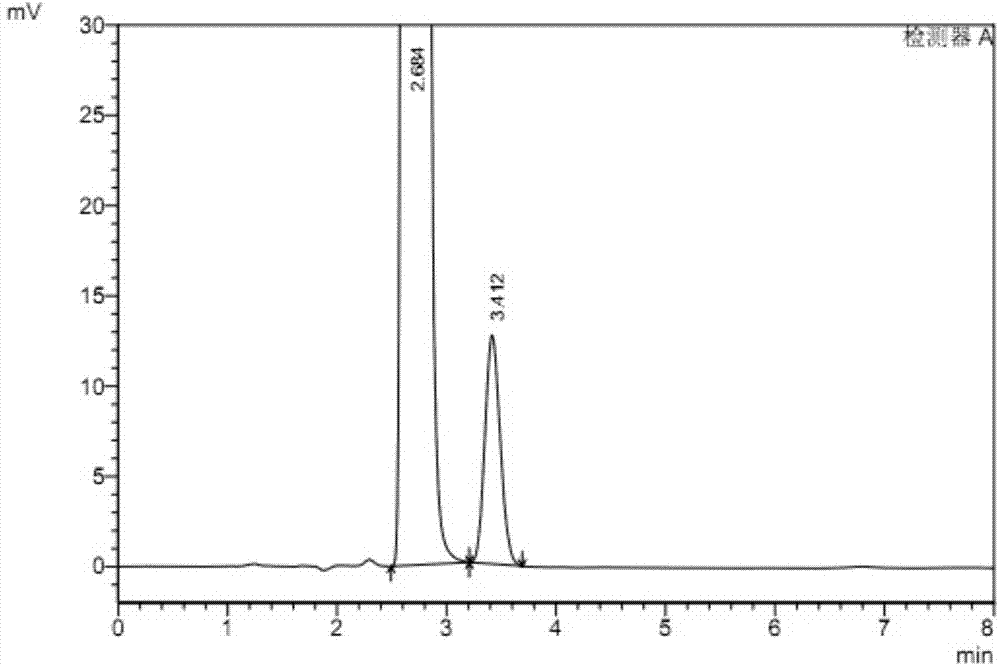
[0049] High performance liquid chromatography detection method for rivaroxaban:
[0050] (1) Preparation of the test solution: Accurately weigh 10 mg of rivaroxaban, place it in a 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution;
[0051] Preparation of reference substance solution: take rivaroxaban R isomer 10mg, put it in a 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark, shake well, and use it as a reference substance stock solution; accurately measure an appropriate amount, and quantitatively dilute it to prepare Each 1ml contains 0.2μg solution, as the reference solution;
[0052] Preparation of system suitability test solution: take rivaroxaban 10mg, put it in a 50ml measuring bottle, add mobile phase to dissolve and dilute to the mark, and shake well to obtain a rivaroxaban solution; take 0.25ml of the reference stock solution, put it in In a 25ml measuring bottle, dilute ...
Embodiment 2
[0071] Except that the mobile phase was changed to acetonitrile-water (40%:60%, volume ratio) in the detection conditions, other steps and conditions were the same as in Example 1.
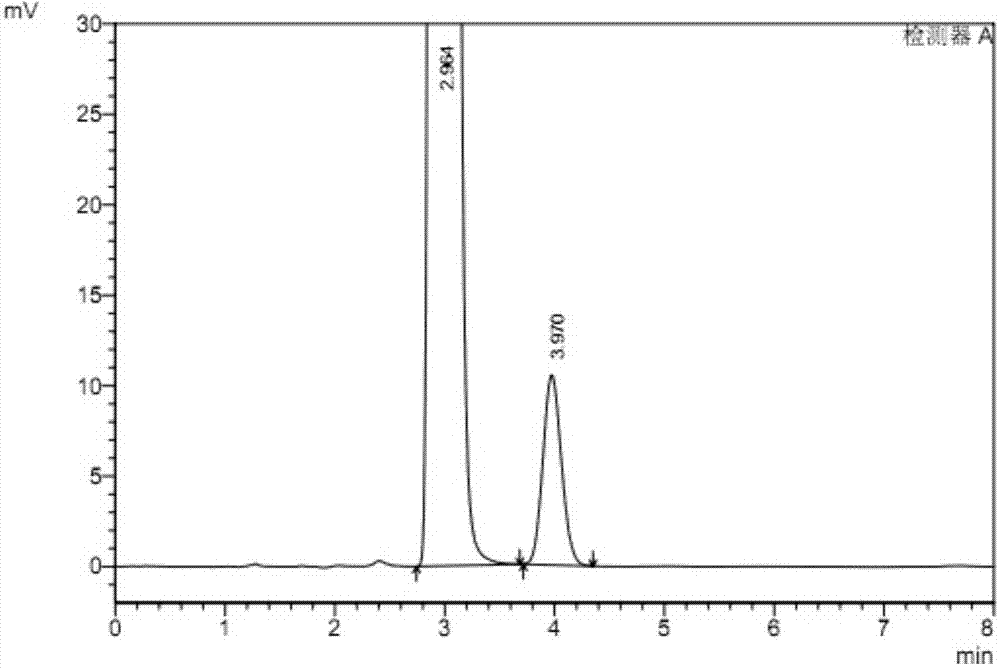
[0072] According to the above detection conditions, take 20 μl of the system suitability test solution and inject it into the liquid chromatograph, record the chromatogram, see figure 2 And table 2; Get reference substance solution 20 μ l and inject liquid chromatograph, adjust detection sensitivity, make the main component chromatographic peak height be about 10% of full scale; Accurately measure each 20 μ l of need testing solution and reference substance solution, inject respectively Liquid chromatograph, record chromatogram, calculate the content of rivaroxaban R isomer in the test sample with peak area by external standard method is 0.02%.
[0073] Table 2, the separation effect of rivaroxaban enantiomers under the conditions of Example 2
[0074]
[0075] The results show that the detec...
Embodiment 3
[0077] Except that the mobile phase was changed to acetonitrile-water (60%:40%, volume ratio) in the detection conditions, other steps and conditions were the same as in Example 1.
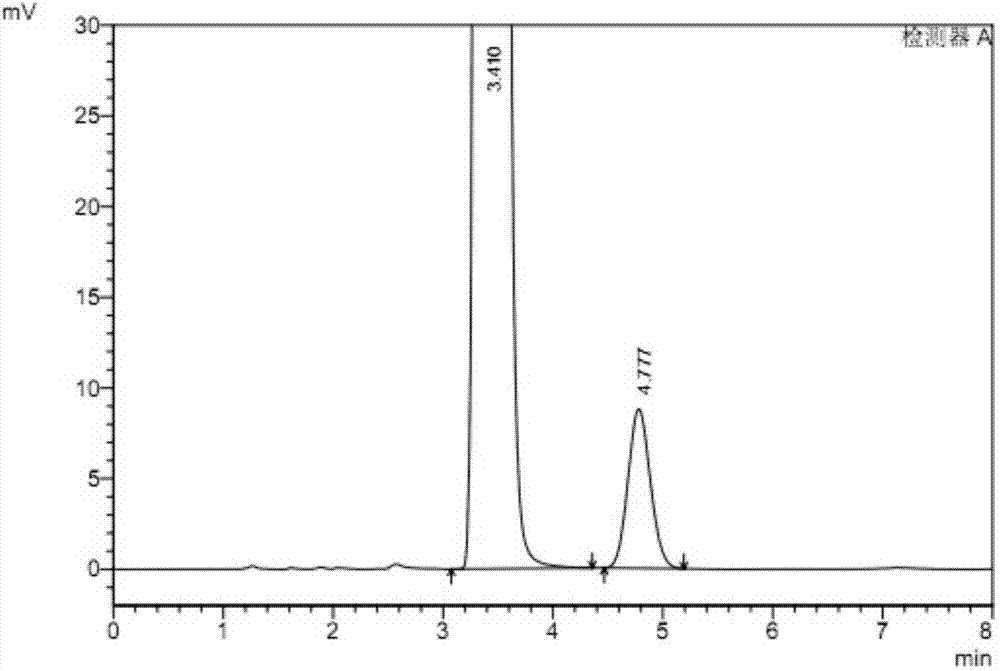
[0078] According to the above detection conditions, take 20 μl of the system suitability test solution and inject it into the liquid chromatograph, record the chromatogram, see image 3 And table 3; Get 20 μ l of reference substance solution and inject liquid chromatograph, adjust detection sensitivity, make the main component chromatographic peak height be about 10% of full scale; Accurately measure each 20 μ l of need testing solution and reference substance solution, inject respectively Liquid chromatograph, record chromatogram, calculate the content of rivaroxaban R isomer in the test sample with peak area by external standard method is 0.02%.
[0079] Table 3, the separation effect of rivaroxaban enantiomers under the conditions of Example 3
[0080]
[0081] The results show that the dete...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com