Preparation method of quinolone medicine passing type solid-phase extraction column
A solid-phase extraction column, quinolone technology, applied in separation methods, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of poor purification effect, difficult to achieve separation and purification, etc., achieve strong interception ability, reduce matrix interference good effect, purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
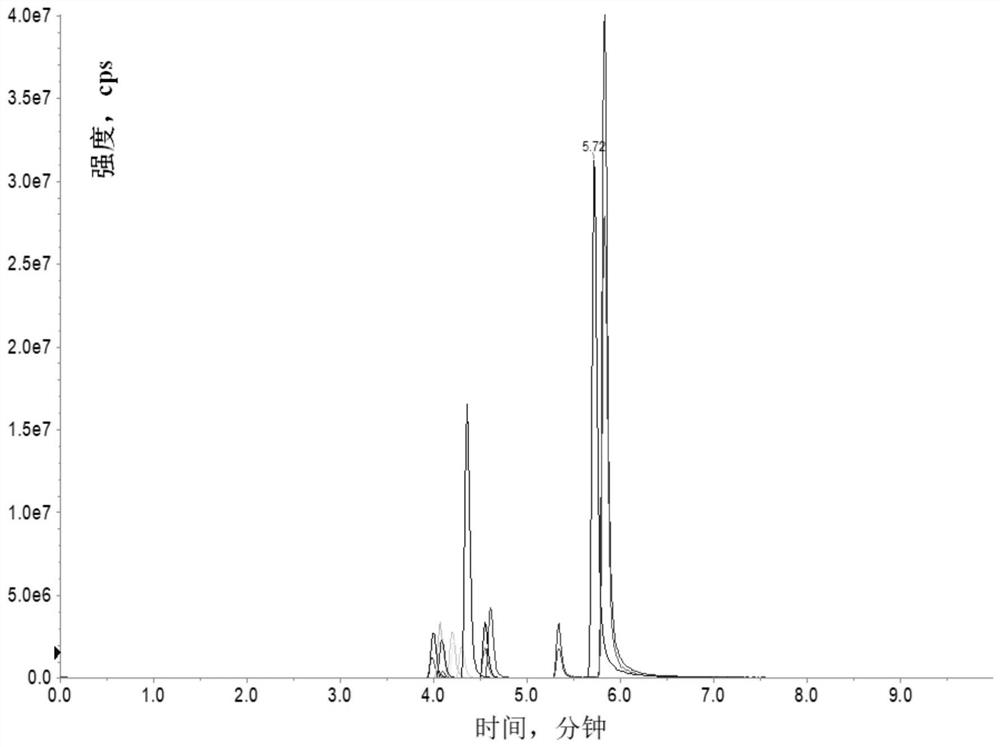
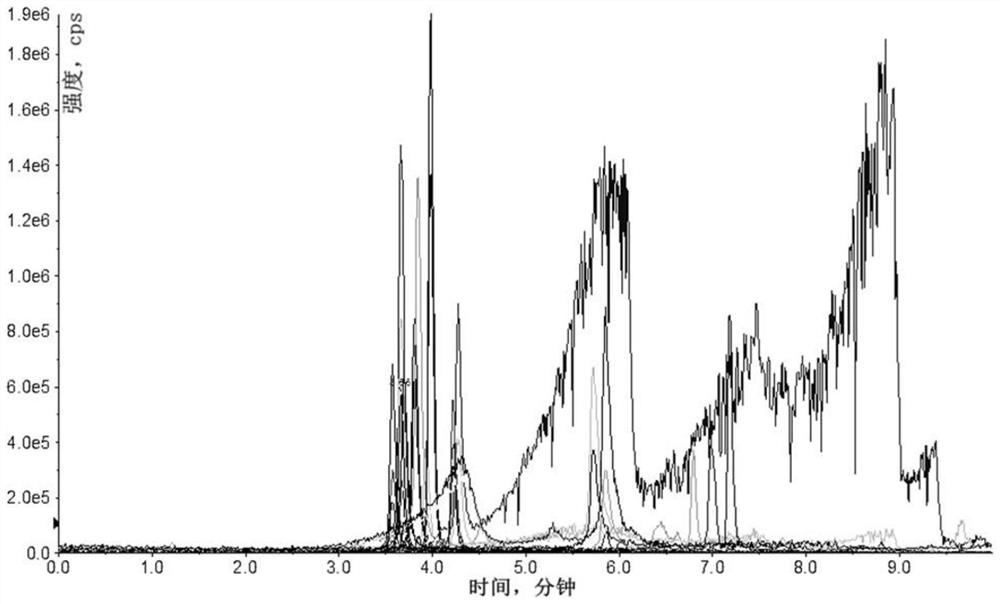
[0038] 1. The preparation method of quinolone drugs provided by the present invention passes the preparation of the solid phase extraction column, including the following steps:
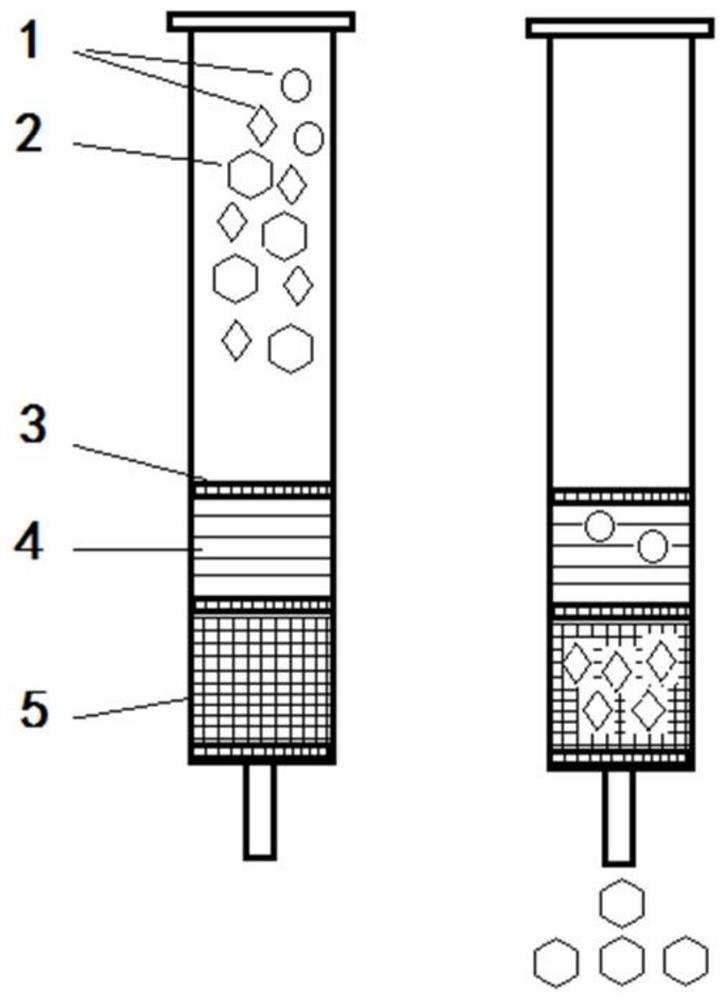
[0039] (1) Mix the spiral COFS composite material to a proper amount of pure organic solvent, ultrasonic treatment to dispersion uniform; fill the pretreated material to the solid phase extracting column of the polypropylene material, and the two ends are blocked by screening;
[0040] The solid phase extraction column is a syringe outer tube having a radial dimension of 1.0 to 2.5 cm. The proportion of spiral COFS composites and pure organic solvents is 50 to 300 mg from: 5 to 20 mL, and then the ultrasonic treatment is 5 to 30 min after 10min.
[0041] (2) Mix the conventional solid phase extraction adsorbent with an appropriate amount of pure organic solvent, ultrasonic treatment to dispersion is uniform; the pre-treated material is continuously filled into step (1) in solid phase extraction column, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| ion source temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com