Method for determining 2beta-methyl-3beta-(4-chlorphenyl) tropine and related substances thereof
A technology of related substances and chlorophenyl, which is applied in the field of content analysis of 2β-carbomethoxy-3β-tropane and related substances, and can solve the problem of inability to detect the quality of β-CCT, inability to detect β-CCT and related substances. Separation and other problems, to achieve the effect of good accuracy, high sensitivity and good separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] β-CXCT, β-CCT, and α-CCT were used to make methanol mixed solution, and the concentrations of β-CXCT, β-CCT, and α-CCT in the mixed solution were 42 μg / mL, 336 μg / mL, and 66 μg / mL, respectively.
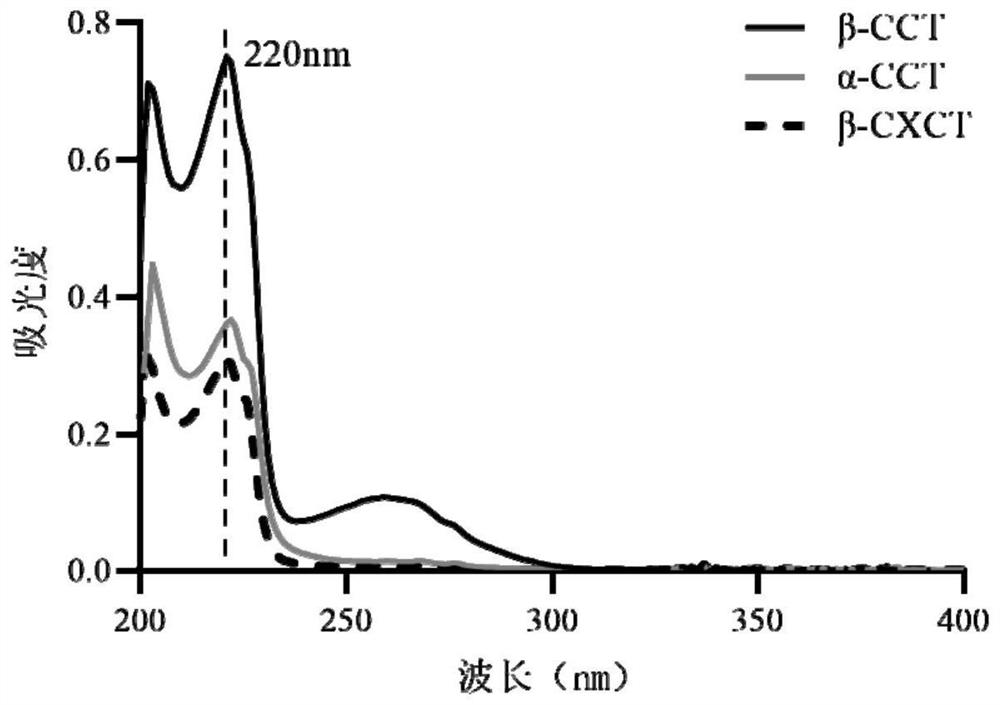
[0057] The mixed solution was separated on a high-performance liquid chromatograph, and the chromatographic column was a C18 column (4.6mm×150 mm, 5 μm, ), the mobile phase was a mixed solution of methanol-water-trifluoroacetic acid in different proportions, the flow rate was 1.0mL / min, the detection wavelength was 220nm, and the column temperature was set at 30°C.
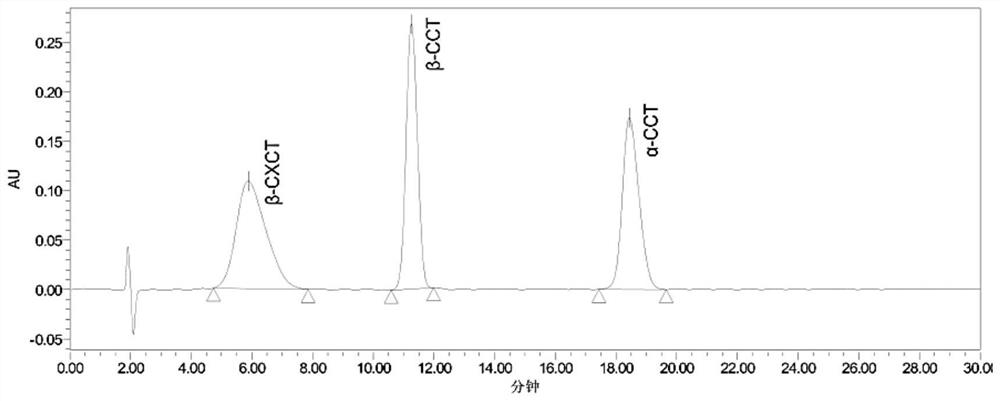
[0058] (1) The ratio of mobile phase methanol-water-trifluoroacetic acid is 25:75:0.1 (v / v / v). Such as figure 2 As shown, the retention times of β-CXCT, β-CCT, and α-CCT are 5.9, 11.3, and 18.4 minutes respectively, and the three can achieve baseline separation, but the peak shape of β-CXCT is relatively broad. The separation degrees of β-CCT, β-CXCT and α-CCT were 4.1 and 8.3, respectively.
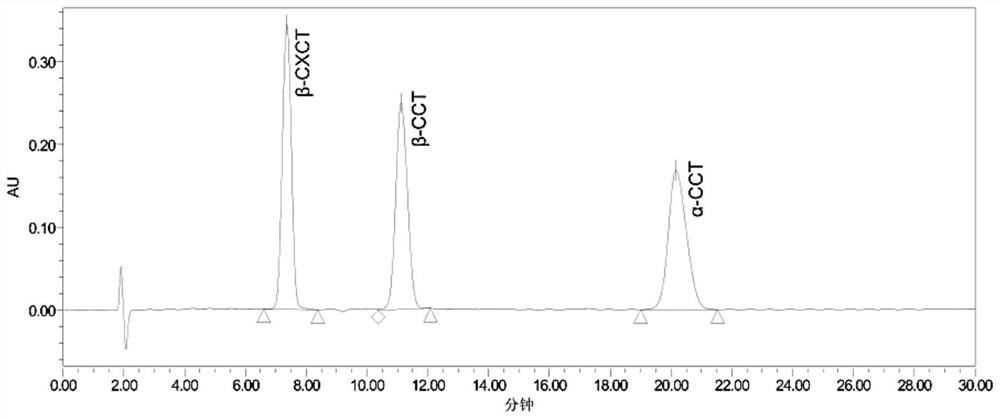
[0059] (2) When...
Embodiment 2
[0062] Embodiment 2 specificity verification
[0063] In order to investigate the specificity of the method of the present invention for β-CCT analysis, that is, to verify that the method provided by the present invention can analyze the impurities that may exist in the sample, the research method (i.e. the international human drug registration technology) Coordination Committee (ICH) recommended method), the degradation test of pure β-CCT under different conditions was carried out, and the samples were processed under the conditions of acid, alkali, oxidation, heating and light respectively. The samples after the test were then analyzed by high performance liquid chromatography.
[0064] The high-performance liquid chromatography analysis conditions are: the chromatographic column is a C18 column (4.6mm×150mm, 5 μm, ), the mobile phase was methanol-water-trifluoroacetic acid (30:70:0.1, v / v / v), the flow rate was 1.0mL / min, the UV detection wavelength was 220nm, and the colu...
Embodiment 3
[0073] Embodiment 3 lowest limit of quantitation
[0074] The lower limit of quantification refers to the concentration of the sample at which the signal number of the chromatographic peak is 10 times that of the noise. First prepare methanol standard solutions of 1.0mg / mL β-CCT, β-CXCT and α-CCT respectively, then dilute them into sample solutions with different concentrations, and inject them into HPLC for analysis. The chromatographic column is a C18 column (4.6mm×150mm, 5 μm, ), the ratio of methanol-water-trifluoroacetic acid is 30:70:0.1 (v / v / v) as the mobile phase, the flow rate is 1.0 ml / mL, and the chromatogram is recorded until the peak height of the sample is 10 times of the noise. The concentration is the lower limit of quantitation. Results The lower limits of quantitation in β-CCT, β-CXCT and α-CCT were 1.5μg / mL, 1.2μg / mL and 1.5μg / mL, respectively. Concentrations above the minimum quantification limit can be accurately measured, and the minimum quantification...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com