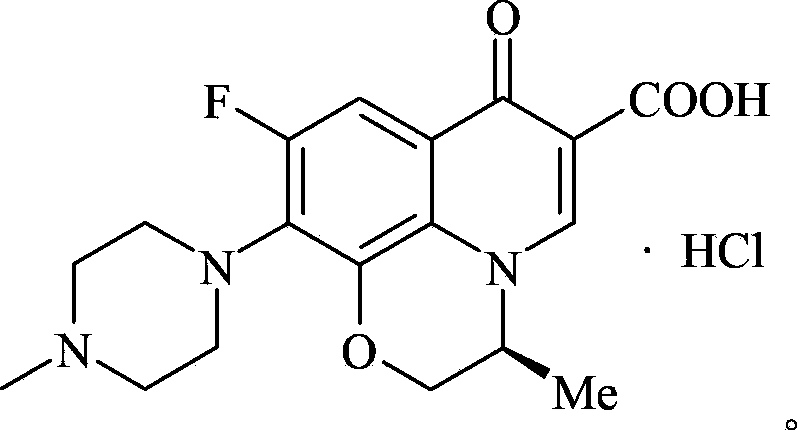
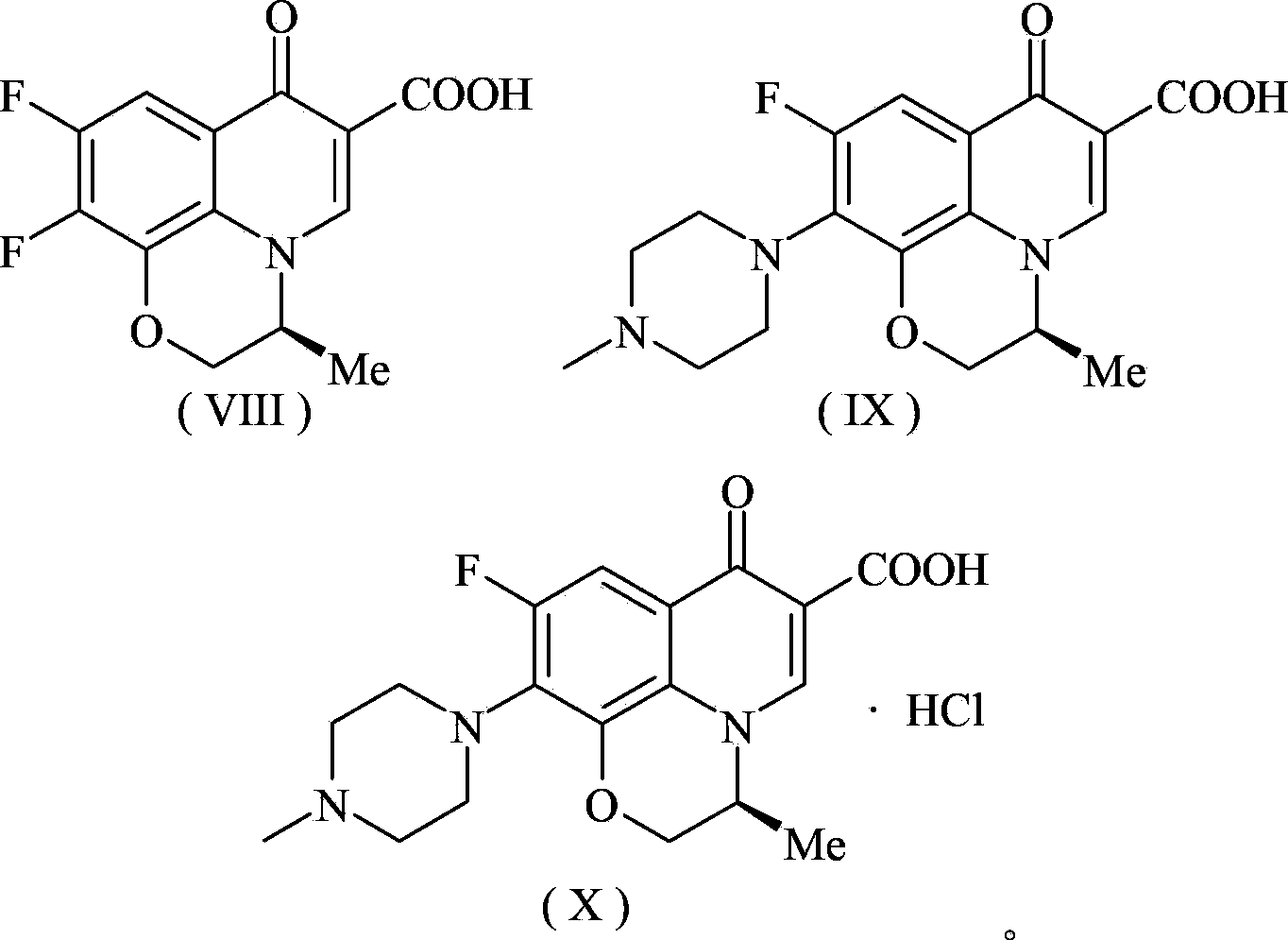
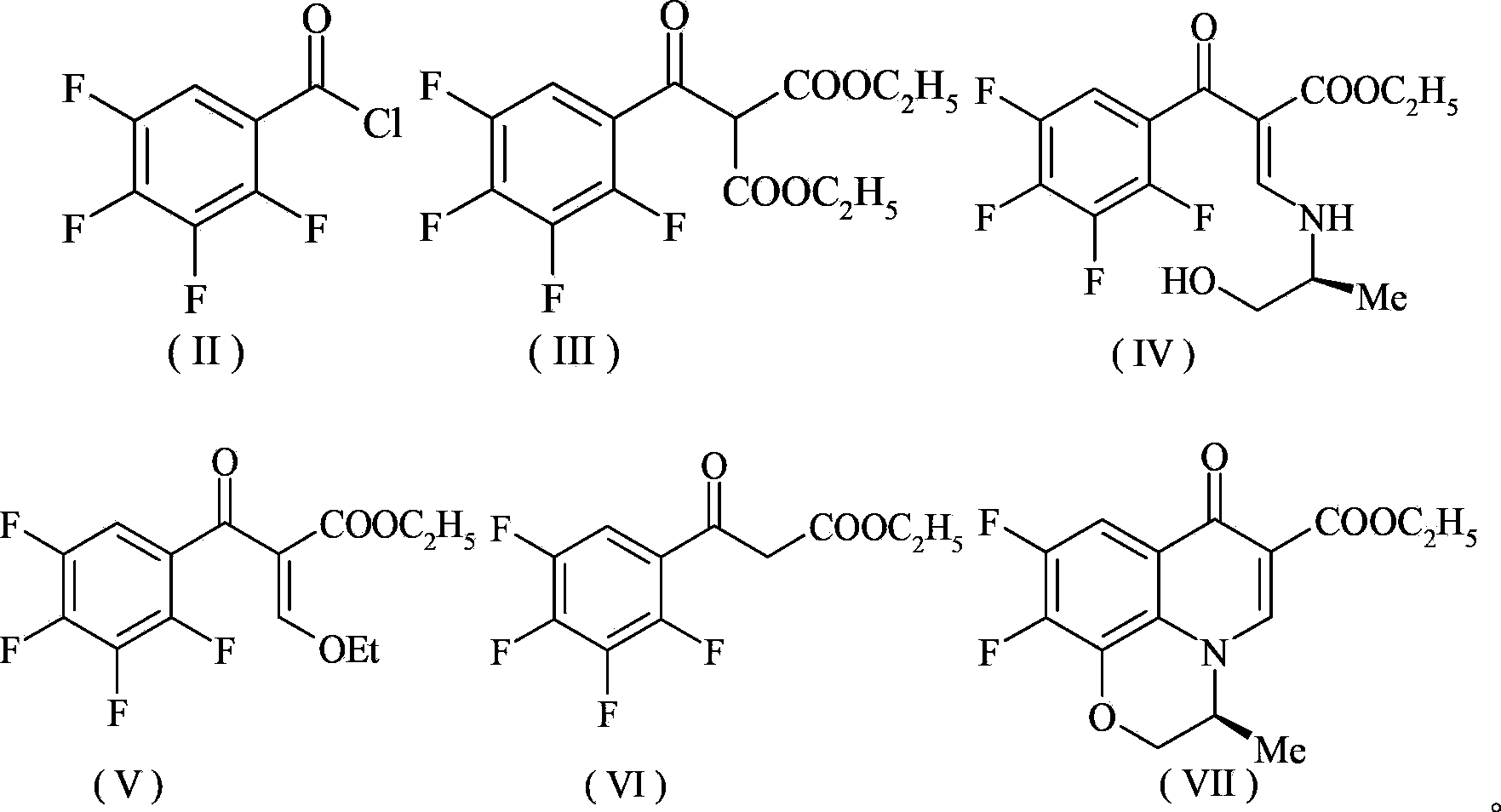
Preparation method of levofloxacin hydrochloride
A technology of levofloxacin hydrochloride and hydrochloric acid, which is applied in the field of medicine, can solve the problems of high catalyst cost, pollution of catalyst recycling environment, cumbersome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] (1)
[0145]
[0146] Under ice-bath conditions, add 500g of 2,3,4,5-tetrafluorobenzoic acid, anhydrous ether (solvent) into a 2-liter reactor, add two droppers of DMF as a catalyst, slowly add oxalyl chloride (whole The dropwise addition process is about half an hour), after the dropwise addition is completed, the reaction is stirred overnight at room temperature. After the completion of the reaction, the solvent was evaporated and the product acid chloride 536g was remembered, and the product yield was 98%.
[0147]
[0148] In a 2-liter reactor, add 530 g of the acid chloride obtained in the previous step reaction, add 1.2 equivalents of diethyl malonate, and then add 5% and 10% of CuI and Fe(AcAc) respectively 3 , DMSO was used as a solvent, and reacted at 40° C. for 5 h. After the reaction was completed, ethyl acetate was added to extract three times, and dried with anhydrous sodium sulfate for 5 hours. The obtained crude product was recrystallized from met...
Embodiment 2
[0164] The solvent for the reaction in step 1 is anhydrous diethyl ether, and the reaction temperature is -5-0°C.
[0165] Step 2 reaction catalytic system is CuI and Fe(AcAc) 3 , the amount of both is 5% and 10% respectively, and the solvent is DMSO.
[0166] The used alkali of step 3 reaction is K 2 CO 3 , the dosage is twice that of the reactant, and the reaction temperature is 120°C.
[0167] The step 4 reaction was stirred overnight at room temperature.
[0168] The solvent used in step 5 reaction is CH 2 Cl 2 , and the reaction was refluxed overnight.
[0169] Step 6 reaction catalytic system is CuI and Fe(AcAc) 3 , the amount of both is 10% and 20% respectively, and the alkali is K 2 CO 3 , the dosage is twice that of the reactants, the solvent is DMF, and the reaction temperature is 100°C.
[0170] The 15% hydrochloric acid solution used in the step 7 reaction was stirred at room temperature for 5 h.
[0171] Step 8 reaction catalyst system is CuI and Fe(AcA...
Embodiment 3
[0174]
[0175] Under ice-bath conditions, add 1.5Kg2,3,4,5-tetrafluorobenzoic acid, anhydrous ether (solvent) to a 6-liter reactor, add six droppers of DMF as a catalyst, and slowly add oxalyl chloride ( The whole dropwise addition process is about half an hour), after the dropwise addition is completed, the reaction is stirred overnight at room temperature. After the completion of the reaction, the solvent was evaporated and the product acid chloride was 1.5Kg, and the product yield was 96%.
[0176]
[0177] In a 6-liter reactor, add 1.5Kg of the acid chloride obtained in the previous step reaction, add 1.2 equivalents of diethyl malonate, and then add 5% and 10% of CuI and Fe(AcAc) respectively 3 , DMSO was used as a solvent, and reacted at 40° C. for 5 h. After the reaction was completed, ethyl acetate was added to extract three times, and dried with anhydrous sodium sulfate for 5 hours. The obtained crude product was recrystallized from methanol to obtain 2.01Kg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com