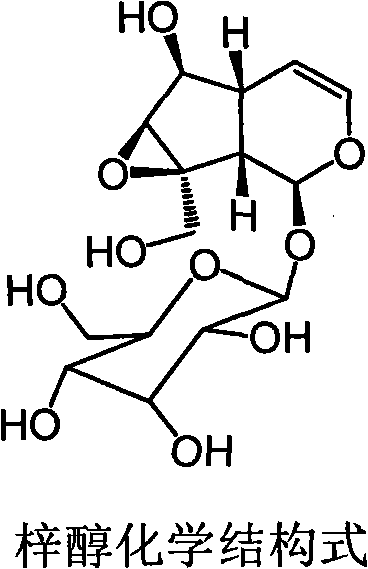
Application of catalpol in preparation of medicine for treating ischemic cerebral apoplexy sequelae
A technology for ischemic stroke and sequelae, applied in the field of medicine, can solve problems such as aggravation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Influence on neurological deficit symptoms
[0018] 1.1 Purpose
[0019] A rat model of permanent cerebral ischemia injury with thread method was used to observe the effect of catalpol on the neurological deficit function of rats with cerebral ischemia injury to determine whether it has the effect of promoting the recovery of sequelae of ischemic stroke.
[0020] 1.2 Test materials
[0021] 1.2.1 Animal health SD male rats weighing 260-290g.
[0022] 1.2.2 Drugs
[0023] 1.2.2.1 Test drug
[0024] Catalpol, white or colorless powder, weighed 0.3g powder, dissolved to 100ml with deionized water, and administered 1ml / 100g body weight by intragastric administration.
[0025] 1.2.2.2 Positive drugs
[0026] Tianbaoning: Produced by Zhejiang Conba Pharmaceutical Co., Ltd., the clinical daily dosage is 6 tablets. Preparation method: Take 7 tablets, grind them into powder and dissolve them to 100ml with deionized water, and administer 1ml / 100g body weight by gavage.
[0027] Edara...
Embodiment 2
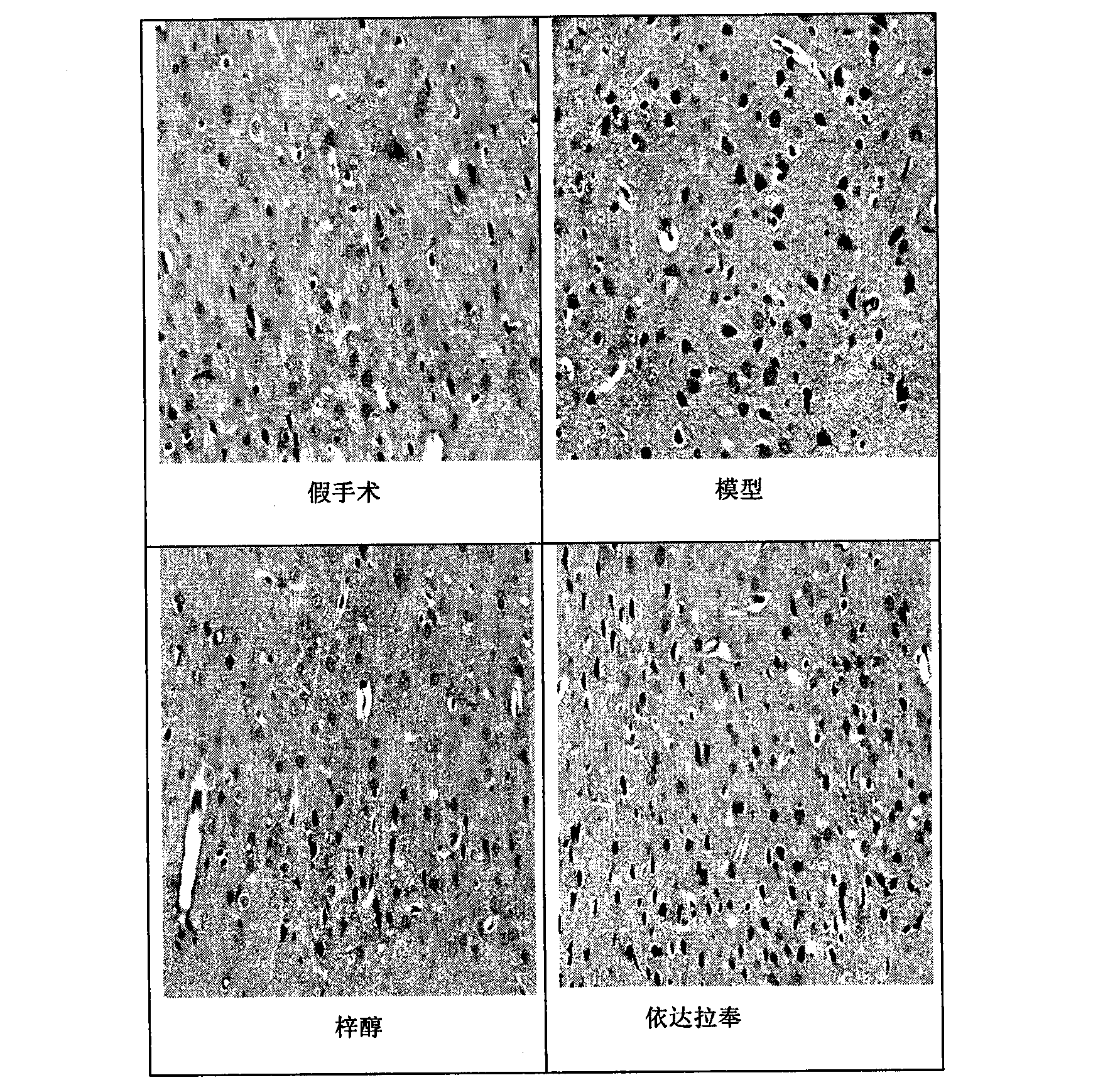
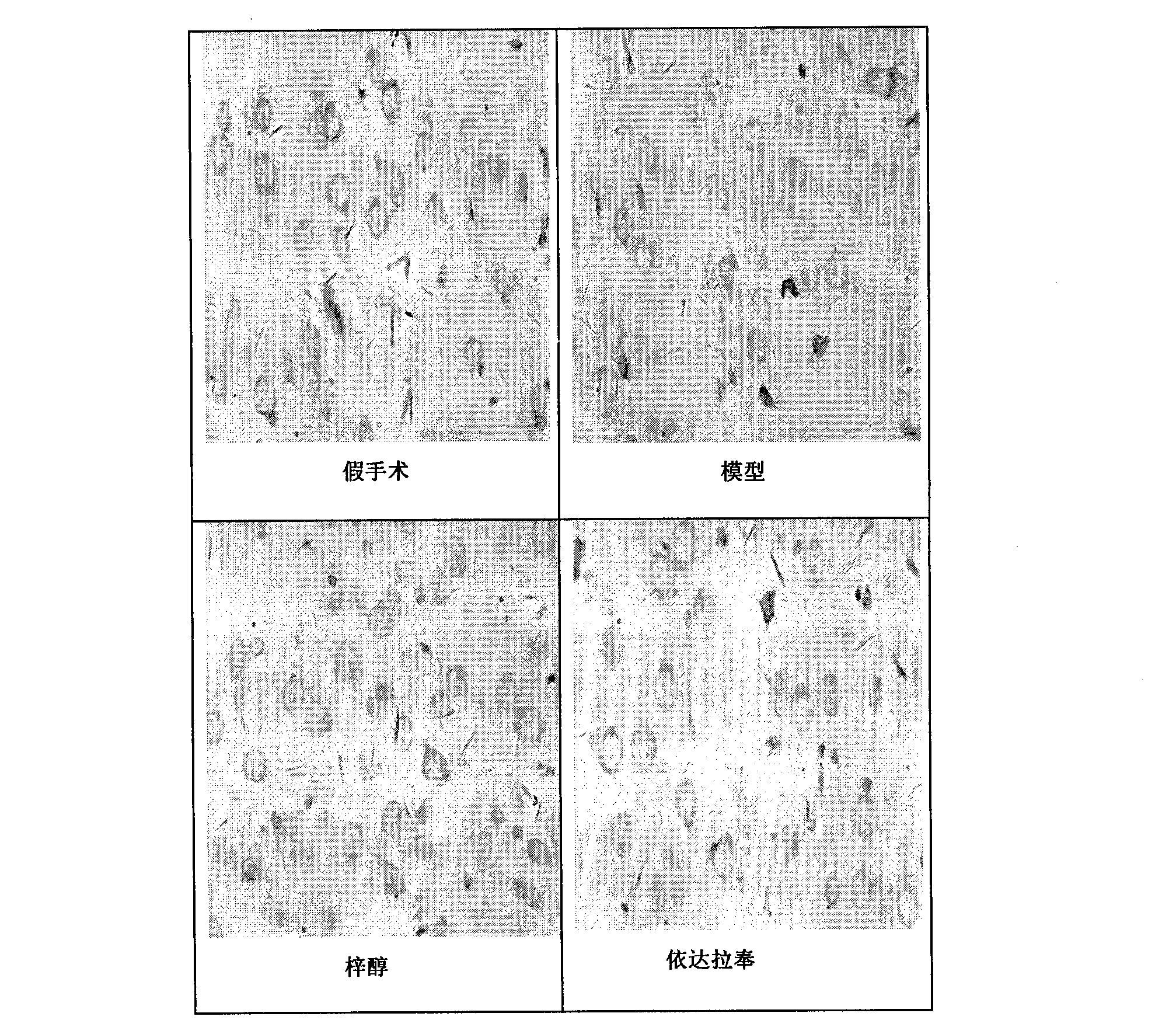
[0066] Example 2 Effect on pathological changes of neurons
[0067] 2.1 Purpose
[0068] To observe the effect of catalpol on the pathological changes of brain tissue neurons in rats with permanent cerebral ischemia injury in the early recovery period, to evaluate the effect of oral administration of catalpol on the early recovery period in rats with permanent cerebral ischemia injury, and whether it can promote neuron survival , Repair and structural integrity.
[0069] 2.2 Test materials
[0070] 2.2.1 Animals
[0071] Same as 1.2.1.
[0072] 2.2.2 Drugs and reagents
[0073] 2.2.2.1 Drugs
[0074] Same as 1.2.2
[0075] 2.2.2.2 Main reagents
[0076] Paraformaldehyde and glutaraldehyde are all domestic reagents and analytically pure.
[0077] 2.2.3 Main experimental equipment
[0078] Biological tissue automatic dehydrator (produced by Hubei Medical Electronic Instrument Factory, TS-12F); LEICA RM2235 rotary paraffin slicer (Germany); biological tissue spreader (produced by Hubei Medical E...
Embodiment 3
[0105] Example 3 Impact on energy metabolism disorders
[0106] 3.1 Purpose
[0107] A rat model of permanent cerebral ischemia injury was used to observe the effect of catalpol on brain energy metabolism disorders in rats with cerebral ischemia injury to determine whether it has the ability to enhance brain energy, reduce acidosis, and improve brain energy metabolism disorders. effect.
[0108] 3.2 Test materials
[0109] 3.2.1 Animals
[0110] Same as 1.2.1.
[0111] 3.2.2 Drugs and reagents
[0112] 3.2.2.1 Test drug
[0113] Same as 1.2.2.1
[0114] 3.2.2.2 Positive drugs
[0115] Same as 1.2.2.2.
[0116] 3.2.2.3 Reagents
[0117] 3.2.2.3.1 Protein, lactic acid (Lac), pyruvate (Pyru) and ATPase kits were purchased from Nanjing Jiancheng Institute of Bioengineering.
[0118] 3.2.2.3.2 Protein, lactic acid (Lac), pyruvate (Pyru) and ATPase kits were purchased from Nanjing Jiancheng Institute of Bioengineering.
[0119] 3.2.3. Main experimental instruments
[0120] HITACHI U-2000 Ultraviolet-V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com