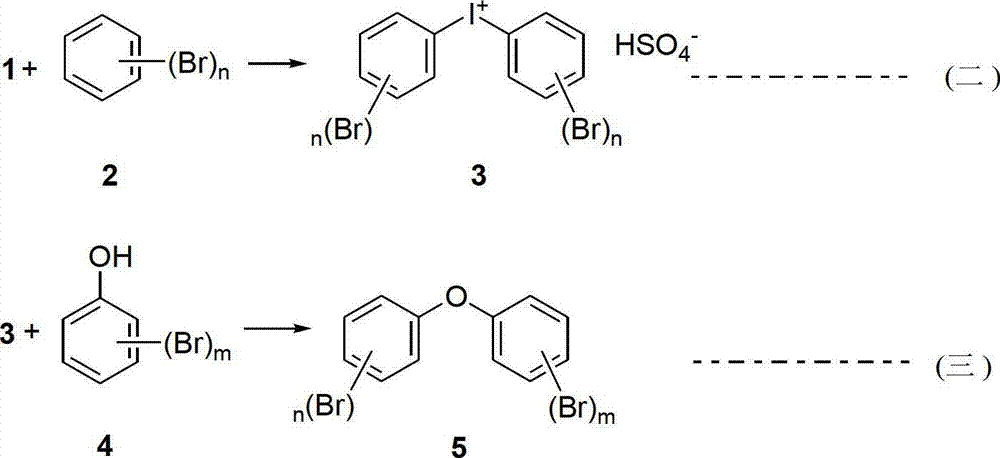
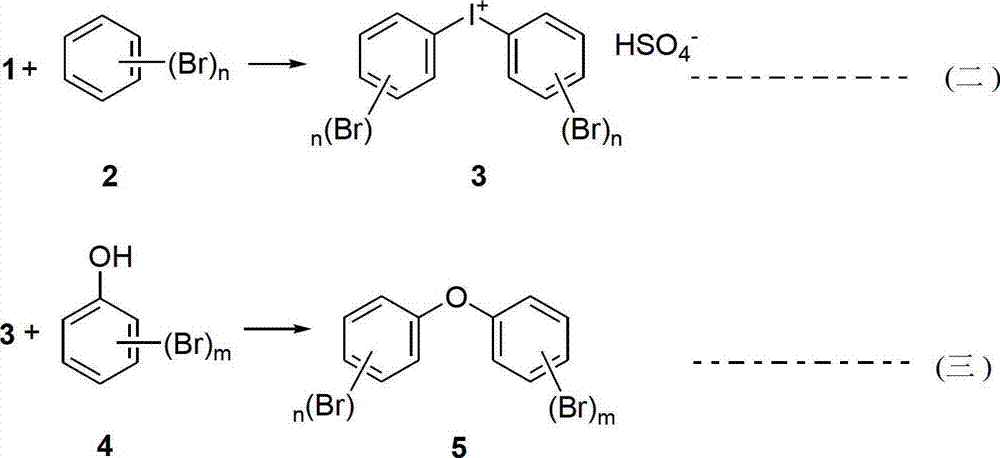
Preparation method of polybrominated biphenyl ether monomer
A technology of polybrominated diphenyl ethers and monomers, applied in ether preparation, organic chemistry, etc., can solve the problems of dangerous experimental operation, difficult preparation and storage, etc., and achieve the effect of simple and safe operation and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of BDE-47
[0027] Compound 1: Suspend iodine (1.0 g, 4 mmol) in 9.0 g of 98% sulfuric acid with a mass concentration of 9.0 g, add sodium iodate (3.2 g, 16 mmol) at 55°C, and complete the addition within 30 minutes. Then react at 55° C. for 2 h to obtain a dark brown reaction liquid, which is directly used in the next reaction without purification.
[0028] Compound 3: Cool the above reaction solution to 20-30°C, dilute with 9.0g mass concentration of 98% sulfuric acid, add 1,3-dibromobenzene (13.1g, 56mmol) within 30min, and react at 20-30°C for 24h. Cool in an ice-water bath to 0-5°C, add 6.0 g of ice water dropwise, collect the solid by suction filtration, wash with 10 mL of water and 10 mL of acetone, and dry naturally in the dark to obtain 4.3 g of a beige solid, 78.2%. .
[0029] BDE-47: Add 80g of dioxane, 40g of water, 2,4-dibromophenol (0.25g, 1mmol), potassium tert-butoxide (0.12g, 1.1mmol) into the reaction flask, stir at room...
Embodiment 2
[0030] Embodiment 2: the preparation of BDE-28
[0031] The preparation of compound 3 was the same as in Example 1.
[0032] BDE-28: Add 100g of dioxane, 40g of water, p-bromophenol (0.17g, 1mmol), potassium tert-butoxide (0.12g, 1.1mmol) into the reaction flask, stir at room temperature for 15min, then slowly add in 30min Compound 3 (0.76g, 1.1mmol), after completion, the temperature was raised to 75°C, and the reaction was stirred for 2h. The solvent was distilled off under reduced pressure, and the resulting white residue was subjected to column chromatography to obtain 0.33 g of a white solid, 81%. 1 H NMR: (600MHz, CDCl 3 )7.78(d,1H,J=1.8Hz),7.44(dd,2H,J=1.8and 7.2Hz),7.39(dd,1H,J=2.4and 9.0Hz),6.84(d,1H,J=8.4 Hz),6.83(d,1H,J=7.2Hz),6.82(d,1H,J=7.2Hz); 13 C NMR (150MHz, CDCl 3 ): 155.91, 153.24, 135.96, 131.82, 130.13, 130.95, 121.93, 119.52, 118.64, 118.18, 117.26, 116.14ppm.
Embodiment 3
[0033] Embodiment 3: the preparation of BDE-100
[0034] The preparation of compound 3 was the same as in Example 1.
[0035] BDE-100: Add 90g of dioxane, 45g of water, 2,4,6-tribromophenol (0.33g, 1mmol), potassium tert-butoxide (0.12g, 1.1mmol) into the reaction flask, and stir at room temperature for 30min , and then slowly added compound 3 (0.76g, 1.1mmol) within 30min, and then raised the temperature to 85°C and stirred for 2h. The solvent was distilled off under reduced pressure, and the resulting white residue was subjected to column chromatography to obtain 0.50 g of a white solid, 89%. 1 H NMR: (600MHz, CDCl 3 )7.77-7.79(m,3H), 7.25-7.27(m,1H),6.27(d,1H,J=9.0Hz); 13 C NMR (150MHz, CDCl 3 ): 151.66, 148.73, 136.32, 135.46, 131.56, 119.45, 119.69, 115.32, 115.33, 112.65ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com