Tablet medicine compound containing levetiracetam
A composition and drug technology, applied in the directions of drug combination, drug delivery, medical preparations containing active ingredients, etc., can solve the problems of affecting and affecting the yield of coated tablets, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
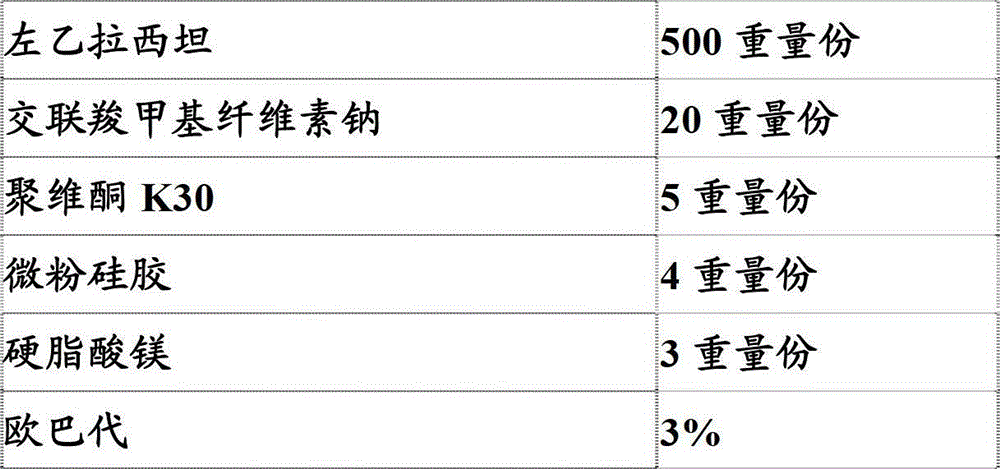
[0293] Embodiment 1: prepare pharmaceutical composition (tablet) of the present invention
[0294] prescription:
[0295]
[0296] Opadry is prepared as a 14% aqueous suspension for use, and 3% in the prescription represents the percentage of the weight of the tablet increased due to coating (ie, the weight of the dry coating material) relative to the total weight of the pharmaceutical composition after coating, which is prepared below The same meaning applies to the examples and comparative examples of the composition.
[0297] Preparation method:
[0298] The method includes the steps of:
[0299] i) fully mixing levetiracetam, disintegrating agent, binding agent, glidant, the mixture obtained is compacted;
[0300] ii) pulverizing the compacted mixture obtained in step i); and
[0301] iii) Add a lubricant to the pulverized mixture obtained in step ii), mix it uniformly, and compress it to form a tablet of the pharmaceutical composition of the present invention, wh...
Embodiment 2
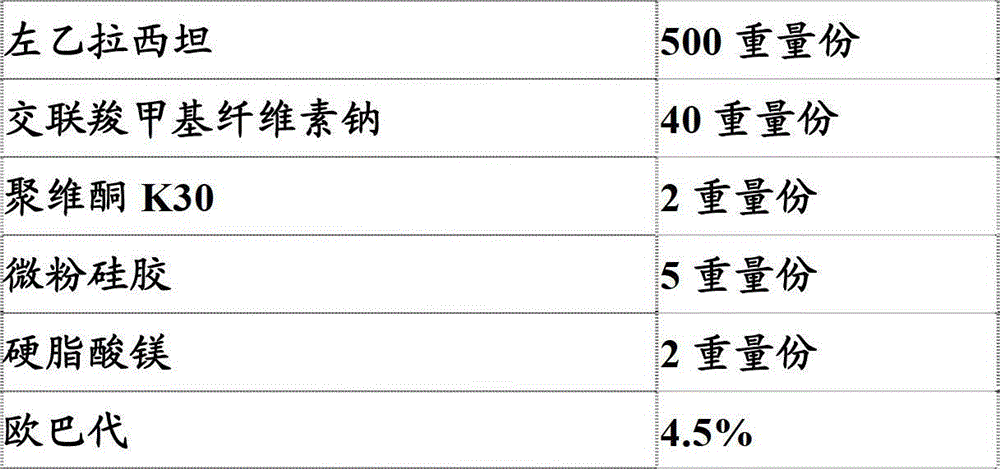
[0303] Embodiment 2: prepare pharmaceutical composition (tablet) of the present invention
[0304] Prescription: with embodiment 1.
[0305] Preparation method:
[0306] i) Levetiracetam, disintegrant, binding agent, glidant are fully mixed;
[0307] ii) adding lubricant;
[0308] iii) fully mixing levetiracetam, disintegrant, binder, glidant and lubricant;
[0309] iv) compacting the mixture obtained in step iii) (in a block);
[0310] v) comminuting the compacted mixture from step iv) (to form particles that pass through a 20-mesh sieve);
[0311] vi) compressing the mixture in step v) to form a tablet of the pharmaceutical composition of the present invention, which may be called a tablet core or plain tablet;
[0312] vii) Coating the tablet obtained in the above steps to obtain a tablet of the pharmaceutical composition of the present invention, which is a coated tablet.
Embodiment 3
[0313] Embodiment 3: prepare pharmaceutical composition (tablet) of the present invention
[0314] Prescription: with embodiment 1.
[0315] Preparation method:
[0316] i) Fully mix levetiracetam, disintegrant and glidant, dissolve the binder with 50% ethanol to make a binder solution, use the binder solution to make soft materials, make wet granules, and dry (measure loss on drying to be less than 3% at 105 ℃ to dry granule), get dry granule;
[0317] ii) adding a lubricant to the pulverized mixture obtained in step i), mixing it uniformly, and compressing it to form a tablet of the pharmaceutical composition of the present invention, which may be called a tablet core or a plain tablet;
[0318] iii) Coating the tablet obtained in the above steps to obtain a tablet of the pharmaceutical composition of the present invention, which is a coated tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com