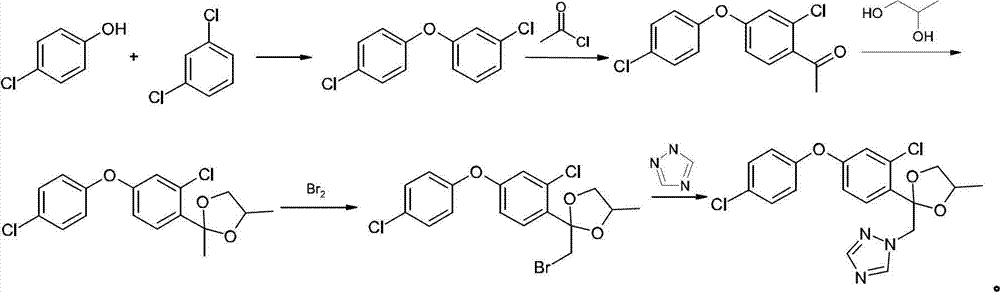
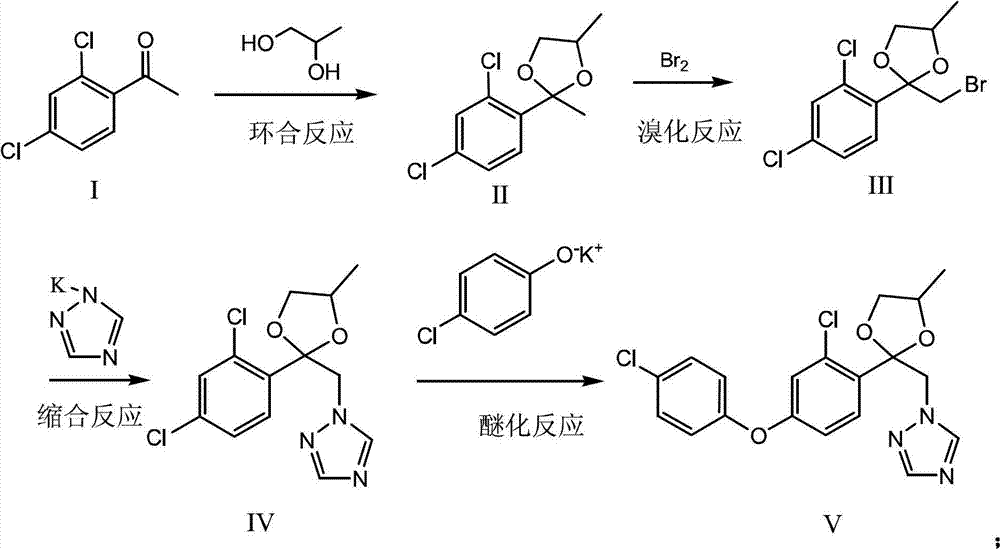
Method for preparing difenoconazole
A technology of difenoconazole and difenoconazole, applied in the direction of organic chemistry and the like, can solve the problems of polluted environment, air pollution, loss and the like, and achieve the effects of lowering reaction temperature, reducing energy consumption and simplifying purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Cyclobromination: 2,4-dichloroacetophenone (1.0mol), 1,2-propanediol (1.0mol) and p-toluenesulfonic acid (2g) are dissolved in cyclohexane (200g), Heat to reflux for water separation, after reflux for 4.0 hours, add 1,2-propanediol (0.1mol) and continue to reflux for water separation. After 1.0 hours of reaction, the reaction ends, cool down to 30°C, add liquid bromine (1.0mol) dropwise, and start When about (0.2mol) is added dropwise, stop the dropwise addition. After the color becomes lighter (initiation is successful), continue to dropwise add the remaining liquid bromine. After the dropwise addition, continue to react for 1.0h. Cyclohexane was recovered to obtain 329 g of brominated ketal with a content of 95% and a yield of 96%.
[0027] (2) Condensation: Dissolve triazole (1.05mol) and potassium hydroxide (1.1mol) in toluene (200g) aqueous solution, reflux and divide water, after reacting for 2.0h, bromide ketal (329g, 0.96mol) Add it to the above reaction so...
Embodiment 2
[0030] (1) Ring closure bromination: 2,4-dichloroacetophenone (1.0mol), 1,2-propanediol (1.0mol) and p-toluenesulfonic acid (2g) were dissolved in toluene (200g), heated to reflux Divide water, after reflux for 4.0 hours, add 1,2-propanediol (0.3mol), continue to reflux and divide water, after 1.0h of reaction, the reaction ends, lower the temperature to 30°C, add liquid bromine (1.0mol) dropwise, and start adding dropwise When it is about (0.2mol), stop the dropwise addition. After the color becomes lighter (initiation is successful), continue to add the remaining liquid bromine dropwise. After the dropwise addition, continue to react for 1.0h. hexane to obtain 331 g of brominated ketal with a content of 94% and a yield of 95.5%.
[0031] (2) Condensation: Dissolve triazole (1.05mol) and potassium hydroxide (1.1mol) in toluene (200g) solution, reflux to separate water, after 2.0h of reaction, bromide ketal (331g, 0.96mol) Add to the above reaction solution, distill toluene, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com