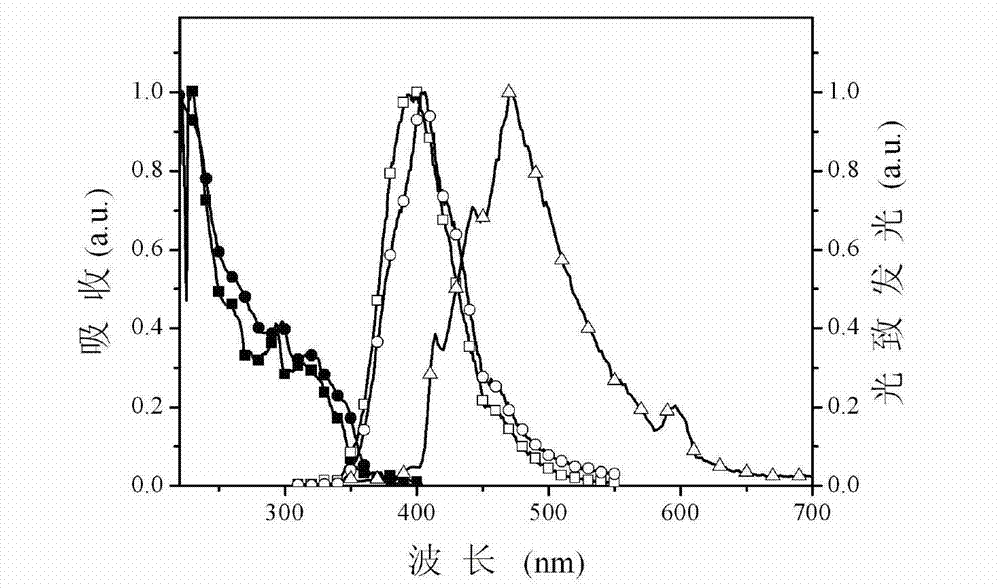
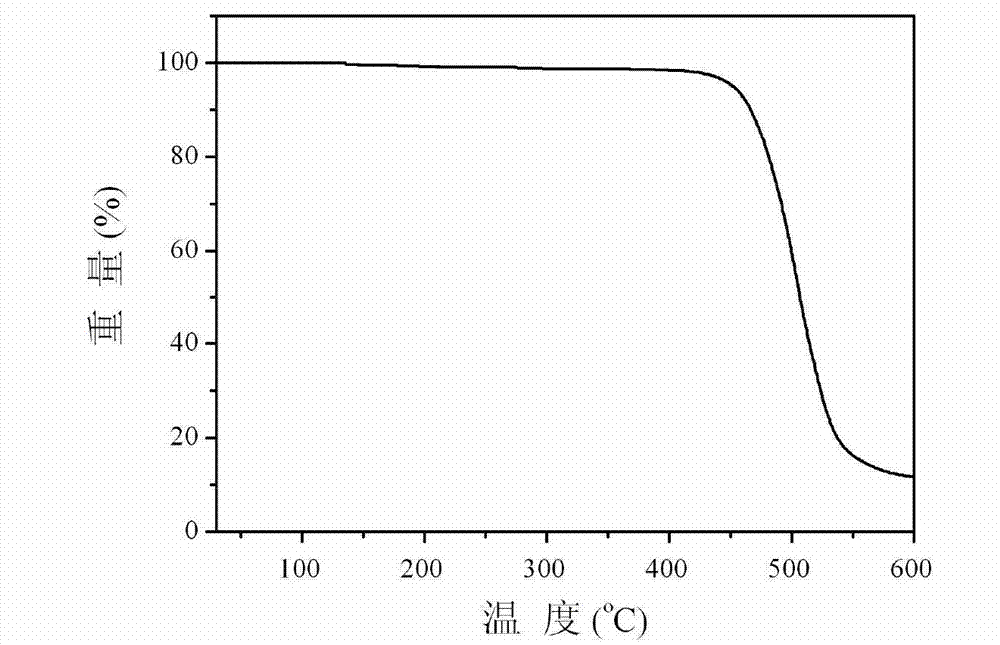
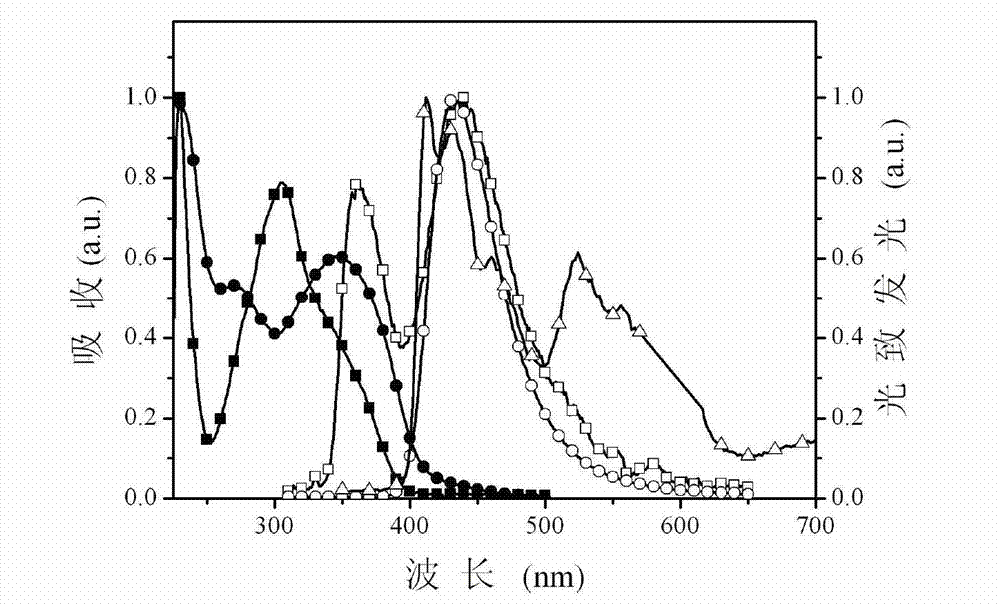
Multifunctional modified phenylate diphosphine oxygen compound and preparation method and application thereof
A technology of diphenylphosphine oxide compound, which is applied in the multifunctional modification of phenyl ether-based bisphosphine oxide compound and the field of preparation and application thereof, can solve the problem of high injection/transmission capability and high starting voltage, and achieve good carrier injection /Transmission capacity, improve overall performance, reduce the effect of starting voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0020] Specific Embodiment 1: This embodiment provides a multifunctional modified phenylene ether-based bisphosphine oxide compound, which is a derivative of a compound with the chemical name 4,6-bis(diphenylphosphinyloxy)phenyl ether. The structural formula of the modified phenyl ether group bisphosphine oxide is:
[0021]
[0022] Wherein, X described in the structural formula, Y structure is as follows:
[0023] (1) X is Y is hydrogen or (2) X is Y is hydrogen or (3) X is Y is hydrogen or (4) X is Y is hydrogen or
[0024] The structural formula of the 4,6-bis(diphenylphosphinoxy)phenyl ether compound described in this embodiment is:
[0025]
[0026] The multifunctional modified phenylene ether-based bisphosphine oxide compound provided by this embodiment can not only maintain a high triplet state energy level, ensure sufficient energy transfer from the host to the guest, but also have good carrier injection / transport capabilities, thereby The goal ...
specific Embodiment approach 2
[0027] Specific embodiment two: The preparation method of the multifunctional modified phenylene ether-based bisphosphine oxide compound provided in this embodiment is specifically completed according to the following steps:
[0028] The preparation method of the multifunctional modified phenylene ether group bisphosphine oxide compound is completed according to the following steps:
[0029] 1. Weigh 4,6-bis(diphenylphosphinooxy)phenyl ether and dissolve it in concentrated sulfuric acid, add N-bromosuccinimide, stir and react at room temperature for 2h~6h, after the reaction, use CH 2 Cl 2 and H 2 O was extracted, the organic phase was dried with anhydrous sodium sulfate, the organic phase solvent was spin-dried, and recrystallized with ethanol to obtain 2-bromo-4,6-bis(diphenylphosphineoxy)phenyl ether or 2,8 -Dibromo-4,6-bis(diphenylphosphinoxy)phenyl ether, wherein the ratio of the volume of concentrated sulfuric acid to the amount of 4,6-bis(diphenylphosphinoxy)phenyl e...
specific Embodiment approach 3
[0042] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the N-bromosuccinimide described in step one and 4,6-bis(diphenylphosphinooxy)phenyl ether The molar ratio is 1:1 or 2:1. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com