Multifunctional modified dibenzofuran-based di-phosphineoxy compound and preparation method and application thereof
A technology of dibenzothienyl bisphosphine and dibenzothiophene, which is applied in the field of multifunctional modified dibenzothienyl bisphosphine oxide and its preparation and application, can solve the problem that the main material of phosphorescent devices cannot simultaneously carry Problems such as carrier injection transmission capacity and high starting voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
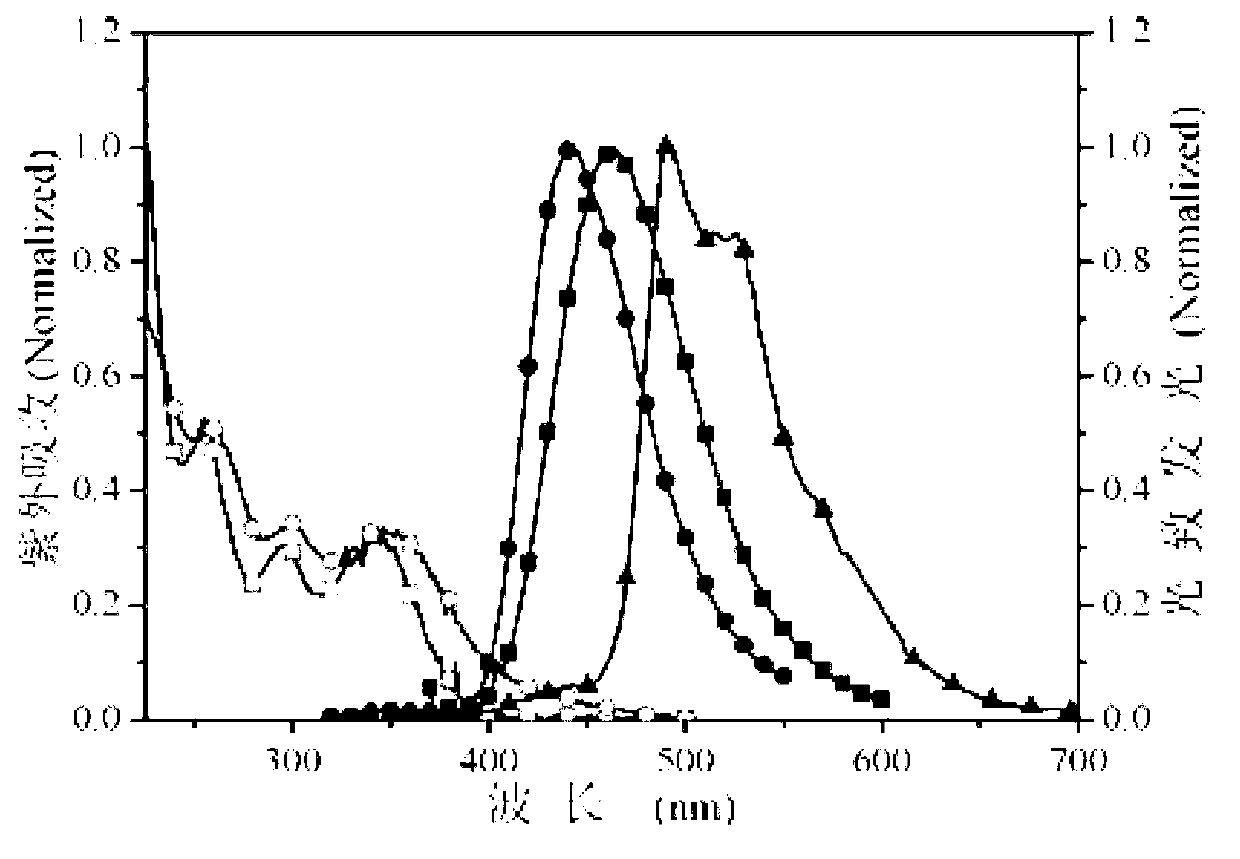
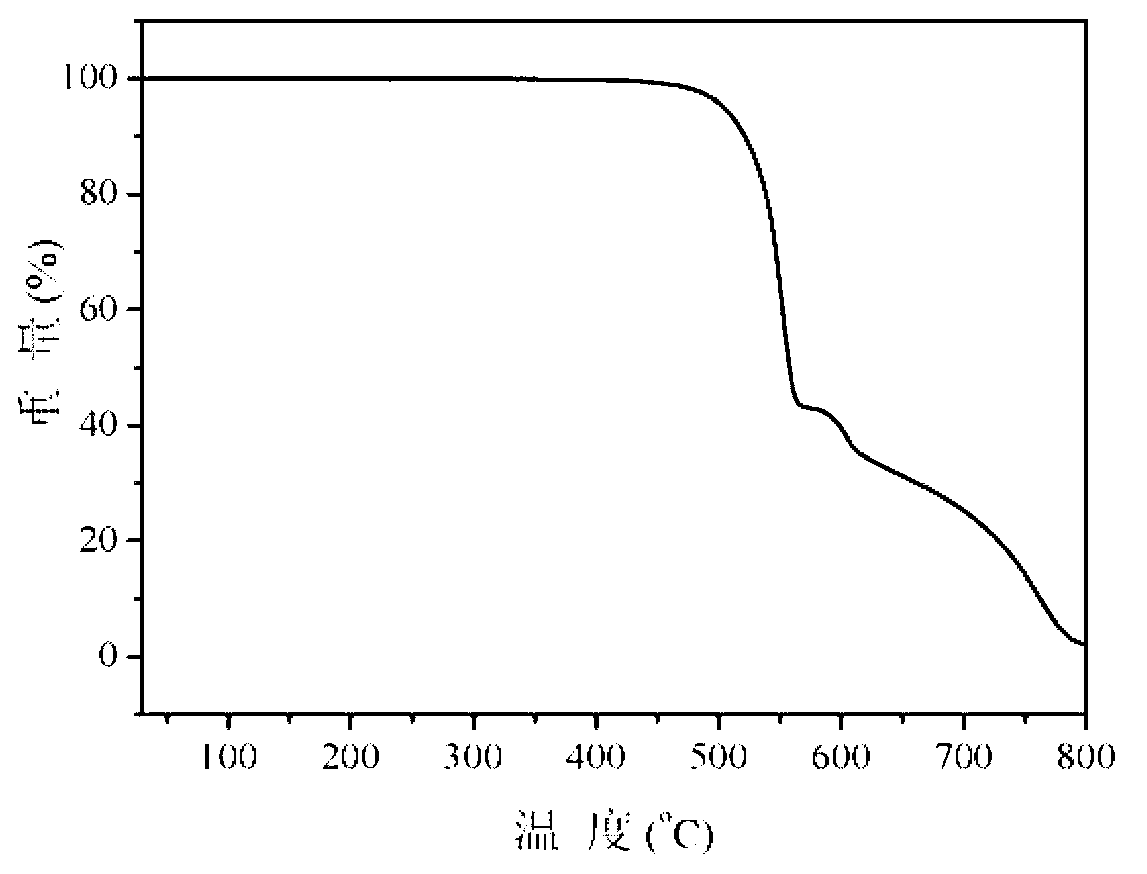
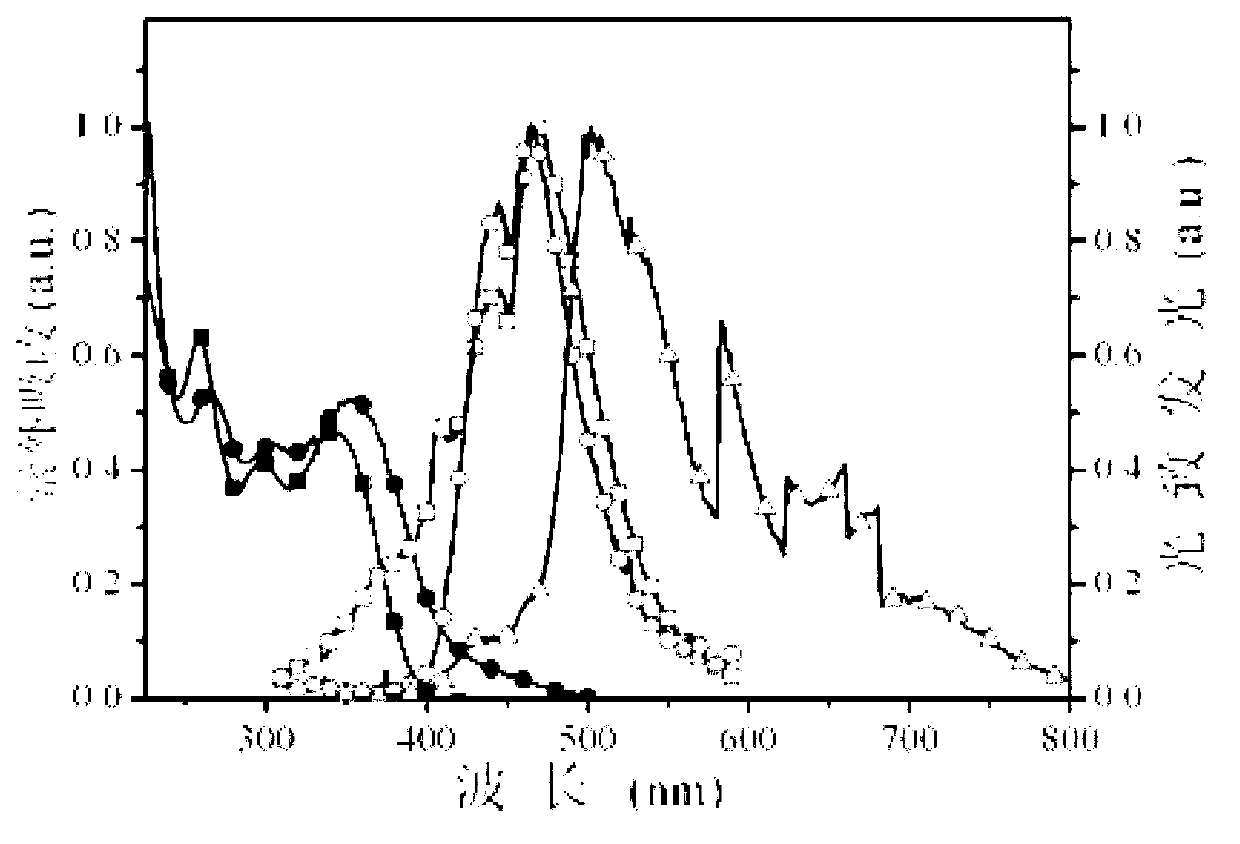
[0013] Specific embodiment 1: This embodiment is a multifunctional modified dibenzothienyl bisphosphine oxide compound, its chemical name is 4,6-bis(diphenylphosphineoxy)dibenzothiophene derivative, and its structure is as follows: Wherein said X, Y are as follows: (1) when X is Y is -H or (2) When X is Y is -H or (3) When X is Y is -H or (4) When X is Y is -H or
specific Embodiment approach 2
[0014] Specific embodiment two: This embodiment is a method for preparing a multifunctional modified dibenzothienyl bisphosphine oxide compound, the chemical name of which is 4,6-bis(diphenylphosphinooxy)dibenzothiophene derivatives. Completed according to the following steps: 1. Bromination: first, 4,6-bis(diphenylphosphinooxy)dibenzothiophene was dissolved in concentrated sulfuric acid, and then N-bromosuccinimide was mixed with 4, The molar ratio of 6-bis(diphenylphosphinooxy)dibenzothiophene is (1~1.2):1. Add N-bromosuccinimide and react with stirring for 2h~4h to obtain the brominated reaction product Using CH 2 Cl 2 and H 2O is extracted, and the obtained brominated organic layer is first dried with a desiccant, then spin-dried by rotary evaporation, and finally purified by column chromatography with ethyl acetate as eluent to obtain 2-bromo-4,6 - bis(diphenylphosphinooxy) dibenzothiophene; 2. Substitution: first add boric acid pinacol ester compound, tetrabutylammoni...
specific Embodiment approach 3
[0022] Specific embodiment three: This embodiment is a method for preparing a multifunctional modified dibenzothienyl bisphosphine oxide compound, the chemical name of which is 4,6-bis(diphenylphosphinooxy)dibenzothiophene derivatives. Completed according to the following steps: 1. Bromination: first, 4,6-bis(diphenylphosphinooxy)dibenzothiophene was dissolved in concentrated sulfuric acid, and then N-bromosuccinimide was mixed with 4, The molar ratio of 6-bis(diphenylphosphinooxy)dibenzothiophene is (2~2.5):1. Add N-bromosuccinimide and react with stirring for 2h~4h to obtain the brominated reaction product Using CH 2 Cl 2 and H 2 O is extracted, and the obtained brominated organic layer is first dried with a desiccant, then spin-dried by rotary evaporation, and finally purified by column chromatography with ethyl acetate as eluent to obtain 2,8-dibromo- 4,6-bis(diphenylphosphinoxy)dibenzothiophene; the ratio of the amount of 4,6-bis(diphenylphosphinoxy)dibenzothiophene su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com