Application of 2-(4-hydroxybenzeneimido)-5-(3-methoxy-4-hydroxybenzylidene)-4-thiazolidone in preparation of antineoplastic drugs
A technology of hydroxybenzylidene group and hydroxybenzylidene group, which is applied in the application field of preparing anti-rhabdomyosarcoma drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of 2-(4-hydroxybenzylidene)-5-(3-methoxy-4-hydroxybenzylidene)-4-thiazolidinone
[0041]
[0042] by HCl:H 2 The ratio of O (1:4) is made into hydrochloric acid solution. Weigh 60mmol of ammonium thiocyanate and dissolve it in 10mL of hydrochloric acid solution, add 40mmol of p-hydroxyaniline, heat to 85°C with stirring, the mixture becomes clear, and monitor the reaction by TLC after reacting for 12h. After the reaction, the mixture was cooled to room temperature and extracted with 10% hydrochloric acid solution to obtain p-hydroxyphenylthiourea.
[0043] Add 6.0mmol of p-hydroxyphenylthiourea and 30mmol of anhydrous sodium acetate to 20mL of ethanol, add 12mmol of ethyl chloroacetate under stirring condition, then heat at 60°C for 6 hours, TLC detects the reaction, after the reaction On cooling, a precipitate appeared. The reaction solution was filtered with suction, the solid was washed with ethanol, and the precipitate was suspended in water to dis...
Embodiment 2
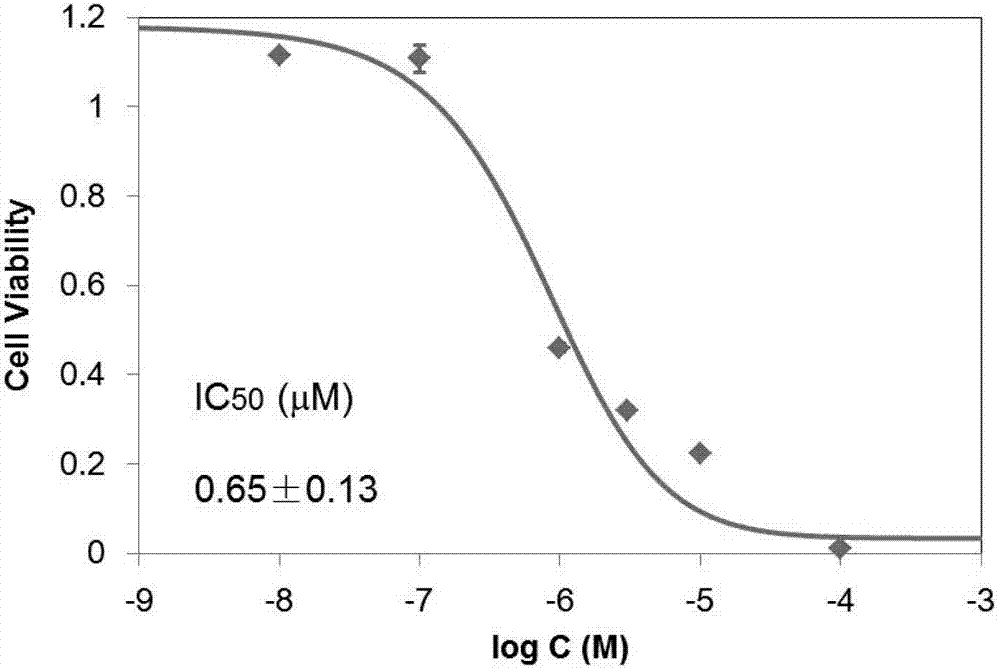
[0046] Example 2 Methods Determination of the half inhibitory concentration of 2-(4-hydroxyphenylimino)-5-(3-methoxy-4-hydroxybenzylidene)-4-thiazolidinone on the survival of RD cells
[0047] 5% CO at 37°C 2 Human embryonic rhabdomyosarcoma RD cells were cultured in DMEM / 10% fetal bovine serum medium under the environment of . The collected log-phase RD cells were seeded in 96-well plates and incubated for 24 h. Add different concentrations (100, 10, 3, 1, 0.1, 0.01 μM) of 2-(4-hydroxyphenylimino)-5-(3-methoxy-4-hydroxybenzylidene)-4- After incubation with thiazolidinone for 48 hours, take it out and place it at room temperature for more than 30 minutes, and let it cool down to room temperature. add to each well Reagent 100 μL, shake the plate for 2 minutes, and let it stand for 10 minutes until the luminous intensity is stable. The luminescence intensity of each well was measured on a microplate reader. Calculate the survival rate according to the luminescence in...
Embodiment 3
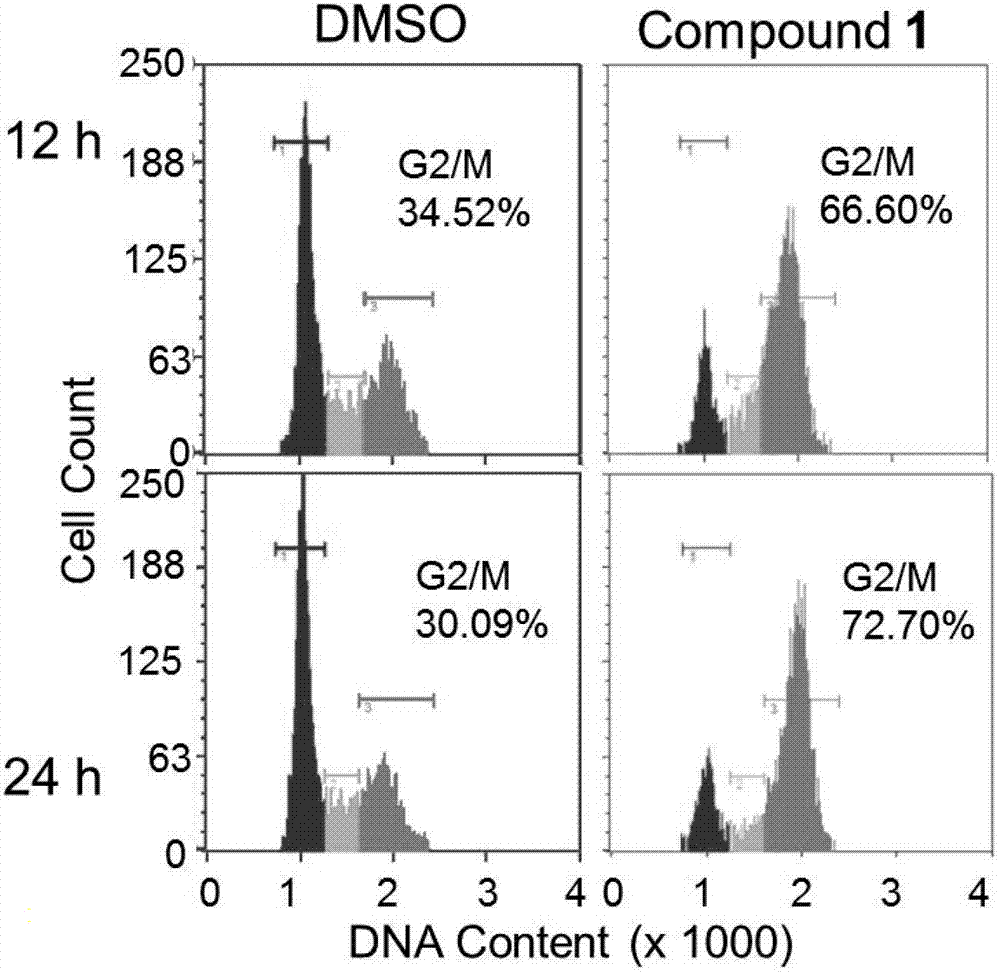
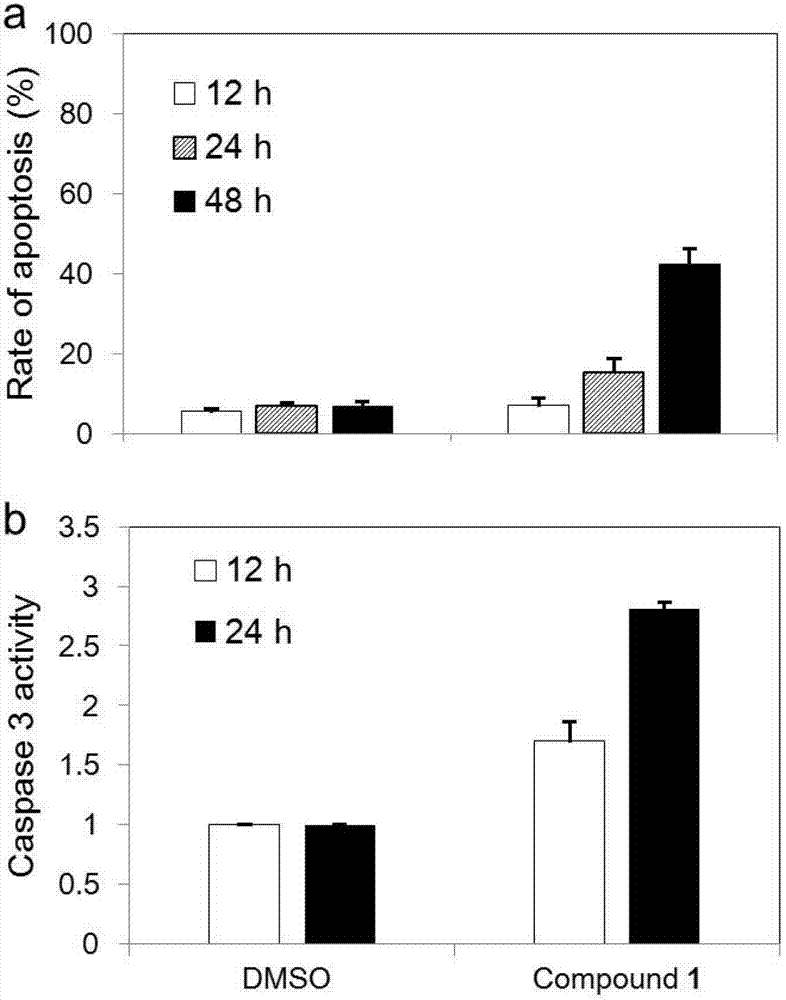
[0050] Effect of 2-(4-hydroxyphenylimino)-5-(3-methoxy-4-hydroxybenzylidene)-4-thiazolidinone on the distribution of RD cell cycle by flow cytometry
[0051] The RD cells in the logarithmic growth phase were inoculated into 60mm culture dishes according to the number of 200,000 cells in 3mL medium volume. After 24 hours, DMSO and 5.0 μM of 2-(4-hydroxybenzylidene)-5-(3-methoxy-4-hydroxybenzylidene)-4-thiazolidinone were added to each 2 dishes . Culture DMSO and 2-(4-hydroxyphenylimino)-5-(3-methoxy-4-hydroxybenzylidene)-4-thiazolidinone cells for 12 h and 24 h, respectively, on ice Digest the cells, collect the cell suspension, centrifuge and wash twice with PBS. Add 10 ml of 70% ice ethanol to suspend and store at -20°C overnight. Centrifuge the cell suspension, wash twice with PBS, add 200 μL of the cell pellet Cell cycle reagent, stained at room temperature for 30 minutes in the dark. The DMSO group was used as negative control.
[0052] The results showed that the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com