Regioselective fucosylation modification method of enzymatic nuclear glucoside class medicine
A technology of regioselectivity and fucosylation, which is applied in the direction of fermentation to achieve the effects of easy separation, environmental friendliness and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
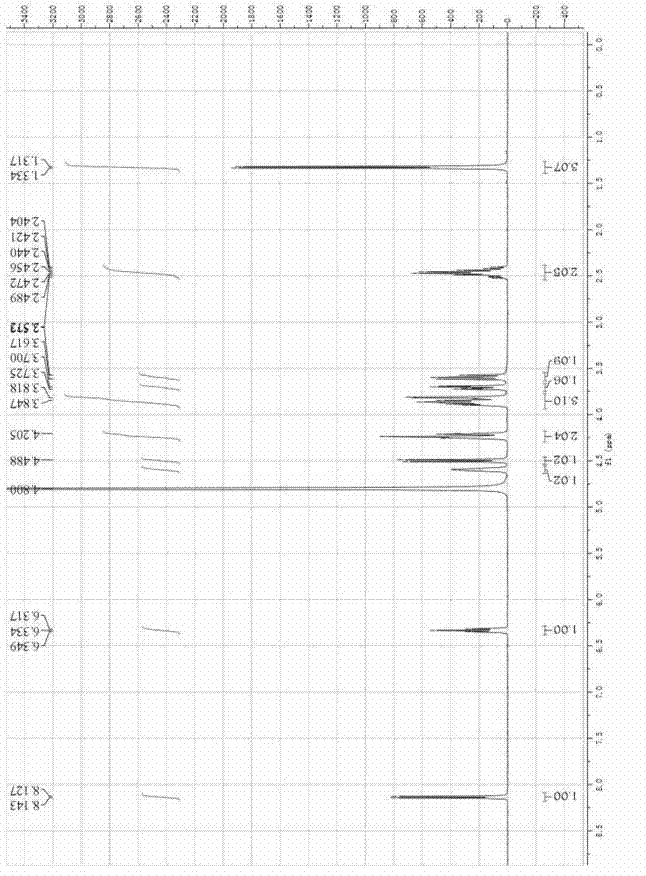
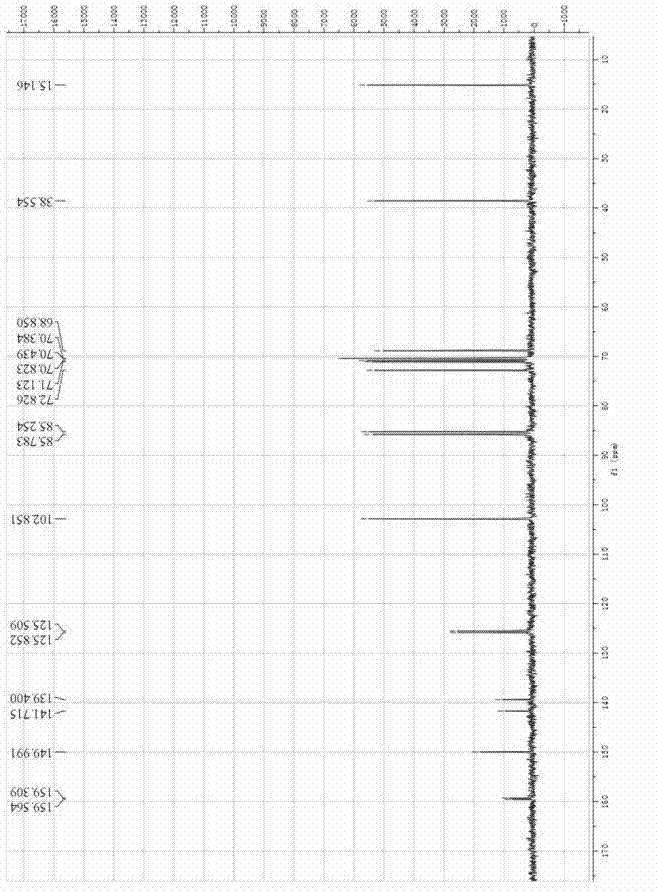
[0037] Add 2mL citric acid-Na into a 15mL Erlenmeyer flask with cap 2 HPO 4 Buffer (50mM, pH 7.0), 0.02mmol p-nitrophenyl β-fucoside and 0.04mmol 5-fluoro-2'-deoxyuridine, mix well, then add 0.06U bovine liver β-galactosidase , React at 40℃ and 200r / min. After 90 hours, the reaction solution was heated to 100°C for 10 minutes to quench the enzyme reaction. After centrifugation, the concentration of the product 5'-O-β-fucosyl-5-fluoro-2'-deoxyuridine was determined by liquid chromatography According to the product calibration curve, the yield is 30%. Product NMR hydrogen spectrum and carbon spectrum are as attached figure 1 with 2 Shown.
Embodiment 2
[0039] Add 2mL citric acid-Na into a 15mL Erlenmeyer flask with cap 2 HPO 4 Buffer (50mM, pH 6.0), 0.02mmol p-nitrophenyl β-fucoside and 0.04mmol 5-fluoro-2'-deoxyuridine, mix well, then add 0.21U bovine liver β-galactosidase , React at 40℃ and 200r / min. After 24 hours, the reaction solution was heated to 100°C for 10 minutes to quench the enzyme reaction. After centrifugation, the concentration of the product 5'-O-β-fucosyl-5-fluoro-2'-deoxyuridine was determined by liquid chromatography According to the product calibration curve, the yield was 41%.
Embodiment 3
[0041] Add 2mL citric acid-Na into a 15mL Erlenmeyer flask with cap 2 HPO 4 Buffer (50mM, pH 7.0), 0.02mmol p-nitrophenyl β-fucoside and 0.04mmol 5-fluoro-2'-deoxyuridine, mix well, then add 0.21U bovine liver β-galactosidase , React at 50℃ and 200r / min. After 17h, the reaction solution was heated to 100℃ for 10min to quench the enzyme reaction. After centrifugation, the concentration of the product 5'-O-β-fucosyl-5-fluoro-2'-deoxyuridine was determined by liquid chromatography According to the product calibration curve, the yield is 40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com