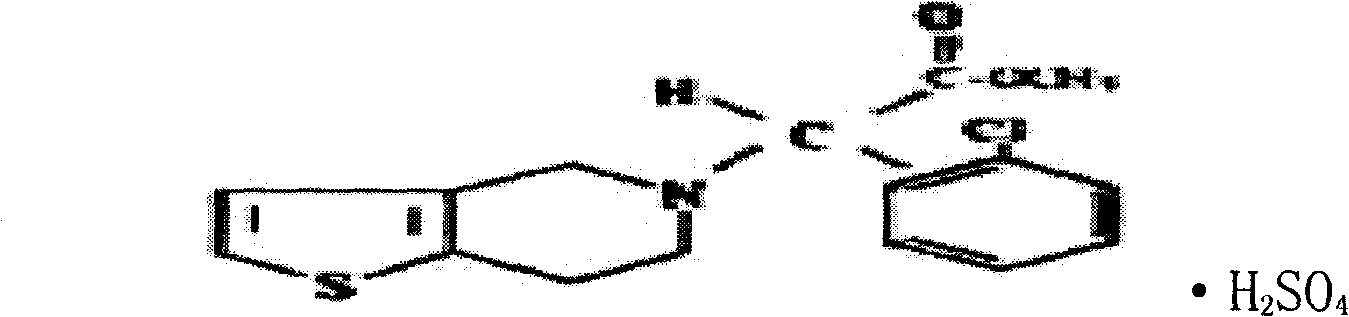
Clopidogrel hydrogen sulfate tablets and preparation method thereof
A technology of clopidogrel bisulfate tablets and clopidogrel bisulfate, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc. Wet process is not feasible and other problems, to achieve the effect of easy operation, simple process, and inhibition of the production of clopidogrel acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
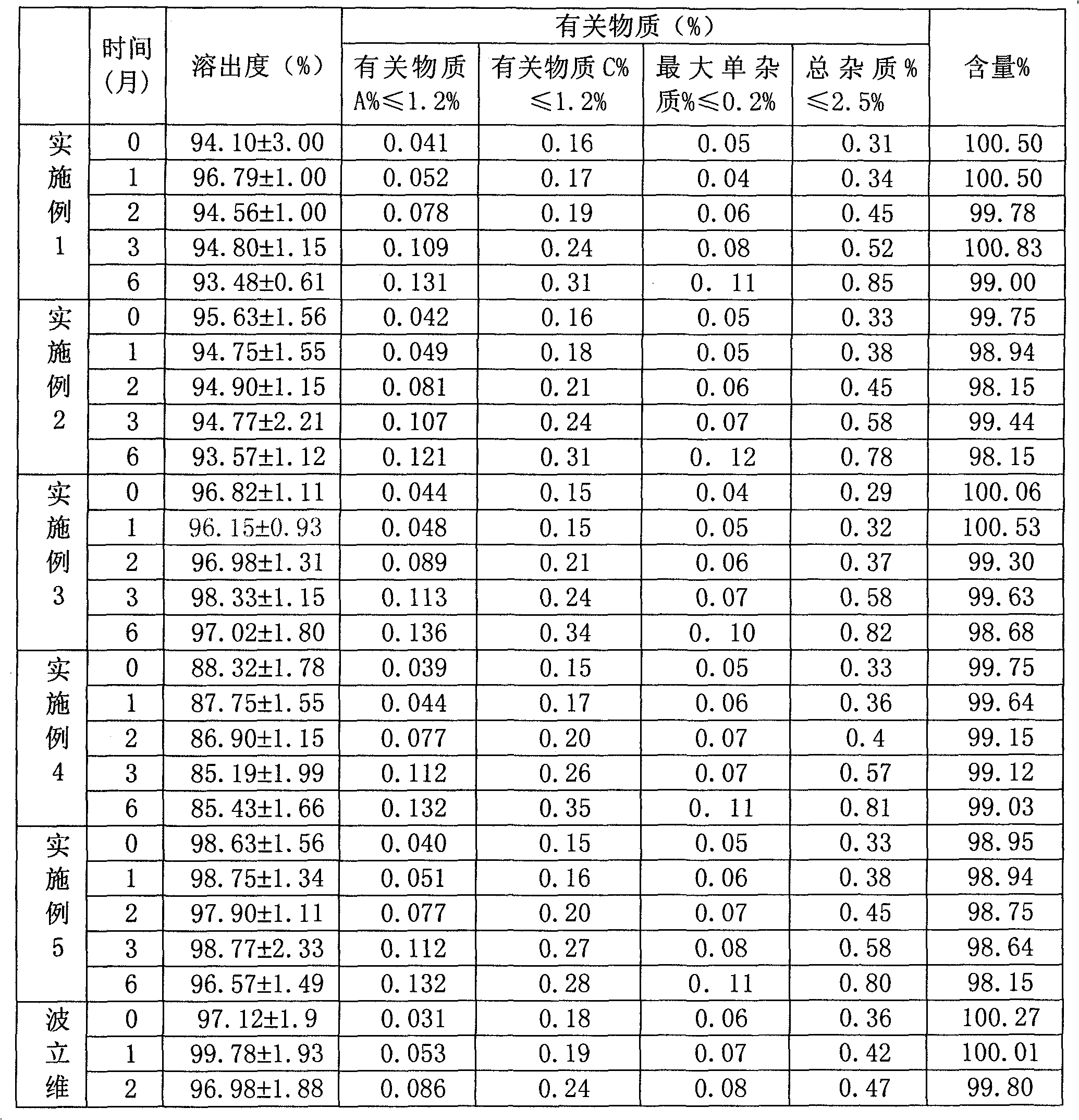
[0045] Prescription for Clopidogrel Bisulfate Tablets:
[0046] Specifications: 75mg (calculated as clopidogrel), tablet weight 300mg, the prescription is as follows:
[0047] Table 1 Prescription of Clopidogrel Bisulfate Tablets 1
[0048] Plain tablet ingredients
Proportion
1000 prescription quantity (g)
32.62%
97.86 (dried to pure)
57.88%
173.64
Hydroxypropyl Methyl Cellulose E501v
3%
9
Low-substituted hydroxypropyl cellulose
4%
12
2%
6
silica
0.5%
1.5
total
100%
1000 pieces
[0049] Clopidogrel bisulfate tablet preparation process
[0050] (1) Various auxiliary materials are passed through a 60-mesh sieve, and the bulk drug of clopidogrel bisulfate is passed through a 120-mesh sieve for subsequent use;
[0051] (2) Weigh 9 g of hydroxypropyl methylcellulose, 12 g...
Embodiment 2
[0057] Prescription for Clopidogrel Bisulfate Tablets:
[0058] Specifications: 75mg (calculated as clopidogrel), tablet weight 300mg, the prescription is as follows:
[0059] Table 2 Clopidogrel bisulfate tablet prescription 2
[0060] Plain tablet ingredients
Proportion
1000 prescription quantity (g)
Clopidogrel bisulfate API
32.62%
97.86 (dried to pure)
56.13%
168.39
Hydroxypropyl Methyl Cellulose E501v
3%
9
Low-substituted hydroxypropyl cellulose
5%
15
3%
9
silica
0.25%
0.75
total
100%
1000 pieces
[0061] Clopidogrel bisulfate tablet preparation process
[0062] (1) Various auxiliary materials are passed through a 60-mesh sieve, and the bulk drug of clopidogrel bisulfate is passed through a 120-mesh sieve for subsequent use;
[0063] (2) Take by weighing 9g of hydroxypropyl methylcellulo...
Embodiment 3
[0069] Clopidogrel Bisulfate Prescription
[0070] Specifications: 75mg (calculated as clopidogrel), tablet weight 300mg, the prescription is as follows:
[0071] Table 3 Prescription of Clopidogrel Bisulfate Tablets 3
[0072] Plain tablet ingredients
Proportion
1000 prescription quantity (g)
Clopidogrel bisulfate API
32.62%
97.86 (dried to pure)
55.18%
165.54
Hydroxypropyl Methyl Cellulose E501v
2%
6
Low-substituted hydroxypropyl cellulose
6%
18
4%
12
silica
0.2%
0.6
total
100%
1000 pieces
[0073] Clopidogrel bisulfate tablet preparation process
[0074] (1) Various auxiliary materials are passed through a 60-mesh sieve, and the bulk drug of clopidogrel bisulfate is passed through a 120-mesh sieve for subsequent use;
[0075] (2) Weigh 6g of hydroxypropyl methylcellulose, 18g of hydroxypro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com