7-oxopyridinopyrimidine compound as well as medicinal composition and application thereof
A technology of oxopyridines and compounds, applied in the field of chemical medicine, can solve the problems that cannot solve the clinical pressure of drug resistance, wild-type cell toxicity and side effects, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
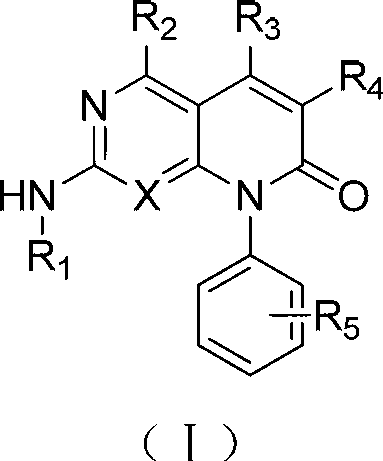
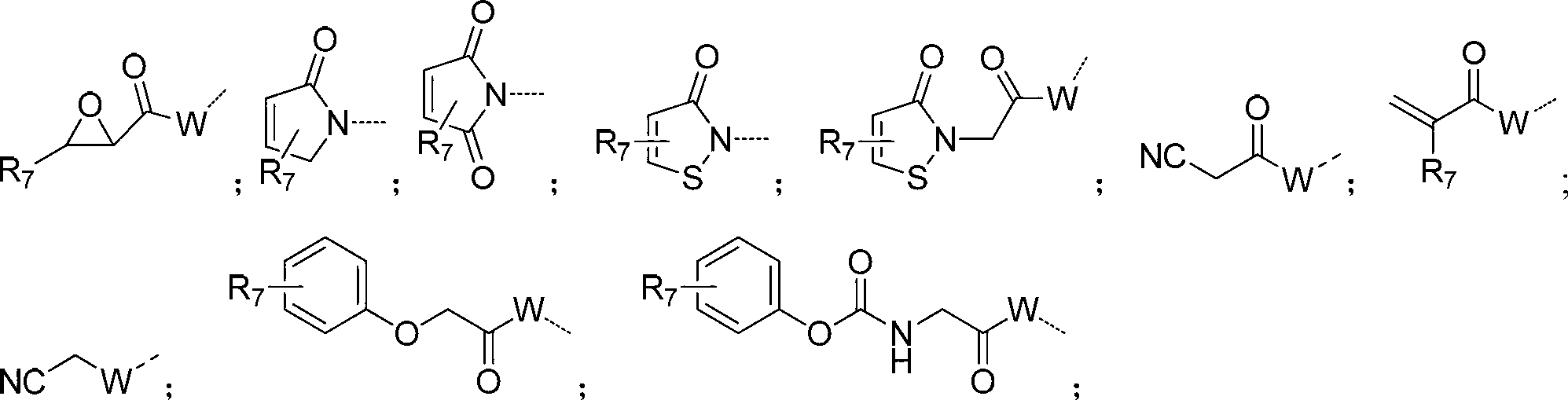
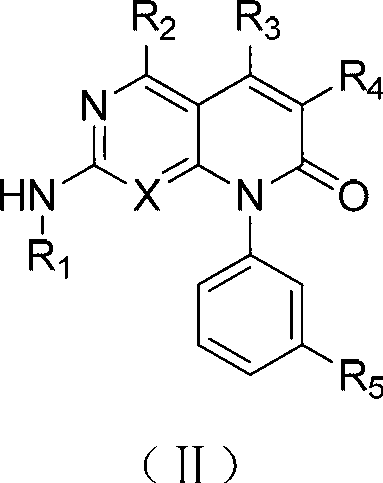
[0192] As the compound of formula I-III described in the invention, those skilled in the art can make 4-chloro-2-methylmercaptopyrimidine-5-ethyl carbonate or 2,4-dichloro -5-Bromopyrimidine was synthesized by several steps reaction as the starting material. (see examples 1, 9, 32, etc. for specific synthesis steps)
[0193] In one embodiment, the present application provides a method for treating transitional proliferative diseases or symptoms such as tumors in humans or other mammals by using the compounds of formulas I-III and pharmaceutically acceptable salts thereof.
[0194] In one embodiment, the compounds designed in this application and their pharmaceutically acceptable salts can be used to treat or control non-small cell lung cancer, small cell lung cancer, lung adenocarcinoma, lung squamous cell carcinoma, pancreatic cancer, breast cancer, prostate cancer , liver cancer, skin cancer, epithelial cell carcinoma, gastrointestinal stromal tumor, leukemia, histiocytic l...
Embodiment 2
[0238] N-(3-(2-((2-methoxy-4-(4-methyl-1-piperazinyl)phenyl)amino)7-oxo-6-phenylpyrido[2,3 -d]pyrimidine-8(7hydrogen)-substituted)phenyl)acrylamide (XTF-251)
[0239]
[0240] The synthesis method is as in Example 1.
[0241] 1 H NMR(400MHz,DMSO)δ10.39(s,1H),8.81(s,1H),8.20(s,1H),8.12(s,1H),7.90(d,J=8.0Hz,1H),7.71 (d,J=7.6Hz,1H),7.66(s,1H),7.53(t,J=8.0Hz,1H),7.43(t,J=8.0Hz,1H),7.36(t,J=7.2Hz ,1H),7.33(d,J=8.8Hz,1H),7.08(d,J=7.6Hz,1H),6.53(d,J=2.0Hz,1H),6.49(dd,J=10.0,16.8Hz ,1H),6.28(dd,J=2.0,16.8Hz,1H),6.04(brs,1H),5.78(dd,J=2.0,10.0,1H),3.78(s,3H),3.06(m,4H ),2.29(m,4H)LCMS(ESI):m / z 588.0[M+H] +
Embodiment 3
[0243] N-(3-(6-benzyl-2-((4-(4-(dimethylamino)-1-piperidinyl)-2-methylphenyl)amino)-7-oxopyrido[ 2,3-d]pyrimidine-8(7hydro)-substituted)phenyl)acrylamide (XTF-230)
[0244]
[0245] The synthesis method is as in Example 1.
[0246] 1 H NMR(400MHz,DMSO)δ10.29(s,1H),8.76(s,1H),8.64(s,1H),7.75(d,J=8.4Hz,1H),7.62(s,1H),7.55 (s,1H),7.42(t,J=8.0Hz,1H),7.30-7.34(m,4H),7.06-7.24(m,1H),7.06(brs,1H),6.95(d,J=7.6 Hz,1H),6.63(s,1H),6.48(dd,J=10.0,16.8Hz,1H),6.28(dd,J=2.0,16.8Hz,1H),5.79(dd,J=10.0,2.0Hz ,1H),3.81(s,2H),3.56(d,J=6.4Hz,2H),2.50-2.57(m,2H),2.12-2.18(m,7H),2.06(s,3H),1.77- 1.80(m,2H),1.38-1.46(m,2H).LCMS(ESI):m / z 614.3[M+H] +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com