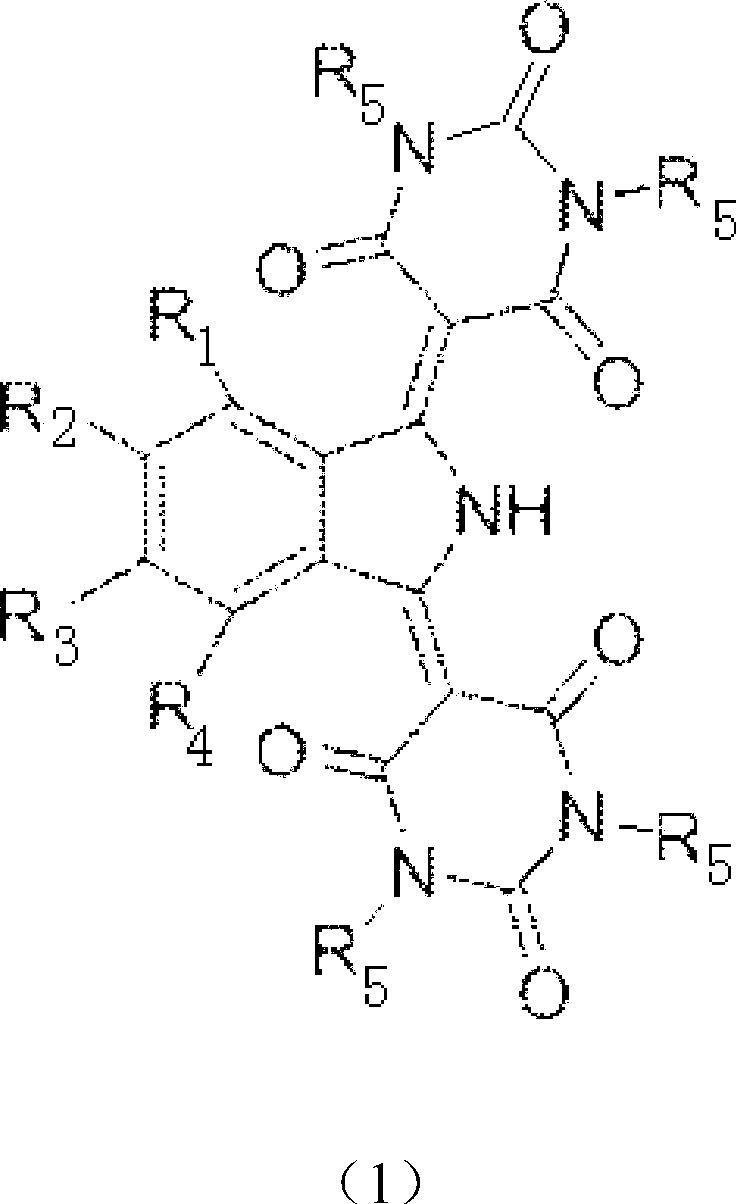
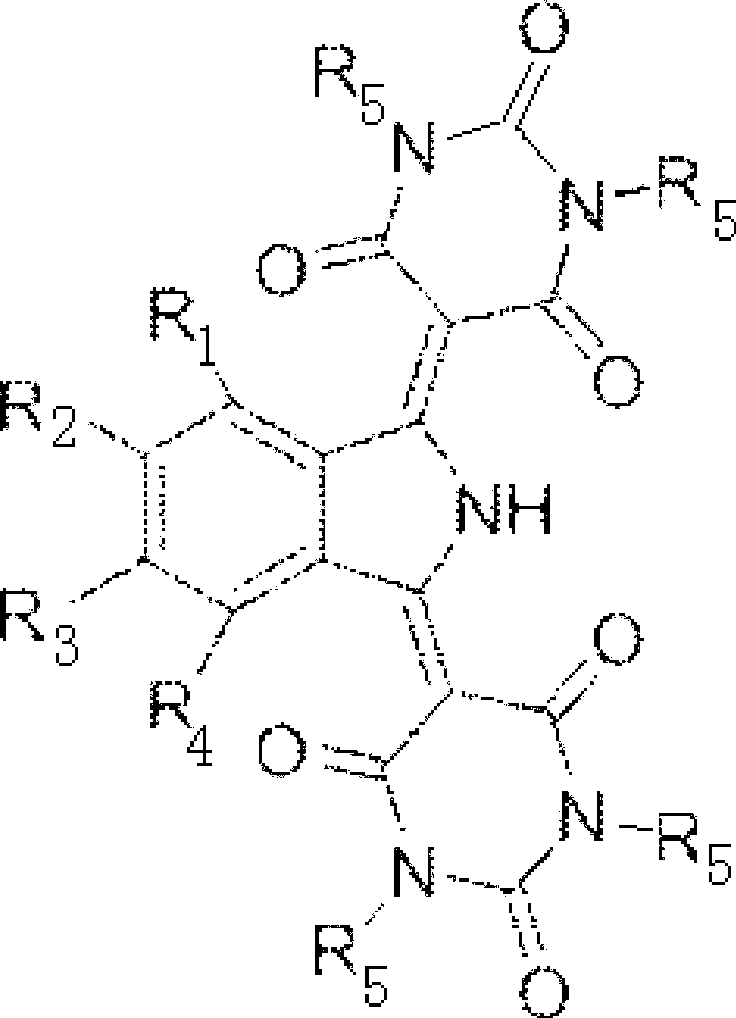
Synthetic method of isoindoline yellow pigment
A technology of indoline yellow and isoindoline is applied in the new synthesis technology field of isoindoline yellow pigment, which can solve the problems of difficult recovery, high recovery cost and high price, and achieves convenient industrial production, cost reduction and post-processing. Difficult to deal with and the effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 3500 g of methanol, 1120 g of phosphoric acid with a weight concentration of 20% in the reaction kettle, then add 256 g (2 moles) of barbituric acid and 190 g (1 mole) of 5-nitro-1,3-diiminoisoindole morphine, heated to 70°C, stirred for 4 hours, filtered, washed with 2000 g of hot water at 80°C, and dried at 110°C for 10 hours to obtain 313.5 g of the yellow pigment represented by formula (1), with a yield of 76%. in:
[0022] R 1 for H, R 2 for NO 2 , R 3 for H, R 4 for H, R 5 for H;
[0023] Wherein: the weight concentration of barbituric acid is 5.05%; The weight concentration of 5-nitro-1,3-diiminoisoindoline is 3.75%; The mol ratio of phosphoric acid and barbituric acid is: phosphoric acid: barbituric acid Bituric acid=1.14:1.
Embodiment 2
[0025] Add 3500 g of methanol, 356 g of phosphoric acid with a weight concentration of 20% in the reaction kettle, then add 256 g (2 moles) of barbituric acid and 190 g (1 mole) of 5-nitro-1,3-diiminoisoindole Phenyl, 20°C, stirred for 16h, filtered, washed with 2000g of hot water at 80°C, and dried at 110°C for 10h to obtain 294.0g of yellow pigment with a yield of 71.4%.
[0026] R 1 for H, R 2 for NO 2 , R 3 for H, R 4 for H, R 5 for H;
[0027] Wherein, the weight concentration of barbituric acid is 5.95%; The weight concentration of 5-nitro-1,3-diiminoisoindoline is 4.42%; The mol ratio of phosphoric acid and barbituric acid is: phosphoric acid: barbituric acid Bituric acid=0.36:1.
Embodiment 3
[0029] With the sulfuric acid of 560g weight concentration 20% the phosphoric acid of replacing 1237g20%, repeat embodiment 1, yield 73%. R 1 for H, R 2 for NO 2 , R 3 for H, R 4 for H, R 5 for H;
[0030] Wherein, the weight concentration of barbituric acid is 5.68%; The weight concentration of 5-nitro-1,3-diiminoisoindoline is 4.22%; The mol ratio of sulfuric acid and barbituric acid is: sulfuric acid: barbituric acid Bituric acid=0.57:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com